First structures of an active bacterial tyrosinase reveal copper plasticity.
Sendovski, M., Kanteev, M., Shuster Ben-Yosef, V., Adir, N., Fishman, A.(2011) J Mol Biol 405: 227-237
- PubMed: 21040728
- DOI: https://doi.org/10.1016/j.jmb.2010.10.048
- Primary Citation of Related Structures:
3NM8, 3NPY, 3NQ0, 3NQ1, 3NQ5, 3NTM - PubMed Abstract:
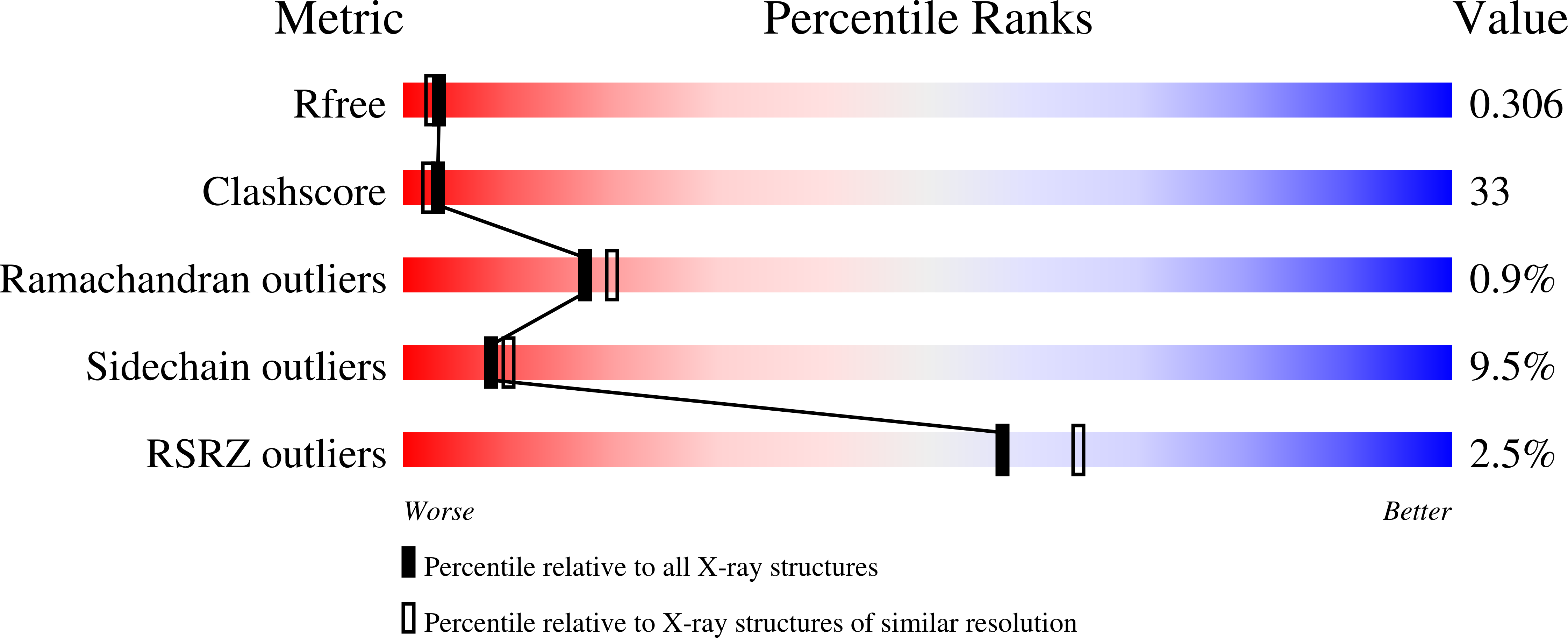
Tyrosinase is a member of the type 3 copper enzyme family that is involved in the production of melanin in a wide range of organisms. The crystal structures of a tyrosinase from Bacillus megaterium were determined at a resolution of 2.0-2.3 Å. The enzyme crystallized as a dimer in the asymmetric unit and was shown to be active in crystal. The overall monomeric structure is similar to that of the monomer of the previously determined tyrosinase from Streptomyces castaneoglobisporus, but it does not contain an accessory Cu-binding "caddie" protein. Two Cu(II) ions, serving as the major cofactors within the active site, are coordinated by six conserved histidine residues. However, determination of structures under different conditions shows varying occupancies and positions of the copper ions. This apparent mobility in copper binding modes indicates that there is a pathway by which copper is accumulated or lost by the enzyme. Additionally, we suggest that residues R209 and V218, situated in a second shell of residues surrounding the active site, play a role in substrate binding orientation based on their flexibility and position. The determination of a structure with the inhibitor kojic acid, the first tyrosinase structure with a bound ligand, revealed additional residues involved in the positioning of substrates in the active site. Comparison of wild-type structures with the structure of the site-specific variant R209H, which possesses a higher monophenolase/diphenolase activity ratio, lends further support to a previously suggested mechanism by which monophenolic substrates dock mainly to CuA.
Organizational Affiliation:
Department of Biotechnology and Food Engineering, Technion-Israel Institute of Technology, Haifa 32000, Israel.