Structural characterization of the stem-stem dimerization interface between prolactin receptor chains complexed with the natural hormone.
van Agthoven, J., Zhang, C., Tallet, E., Raynal, B., Hoos, S., Baron, B., England, P., Goffin, V., Broutin, I.(2010) J Mol Biology 404: 112-126
- PubMed: 20875426
- DOI: https://doi.org/10.1016/j.jmb.2010.09.036
- Primary Citation of Related Structures:
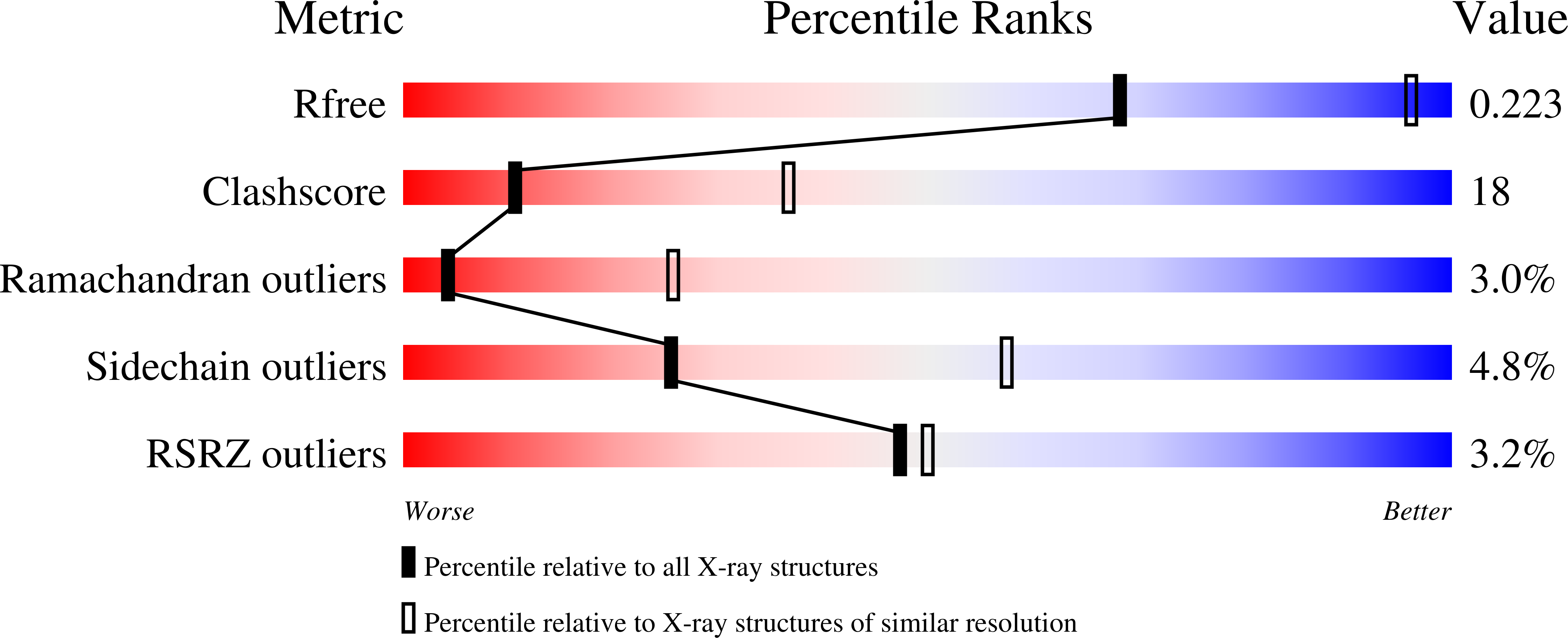
3NPZ - PubMed Abstract:
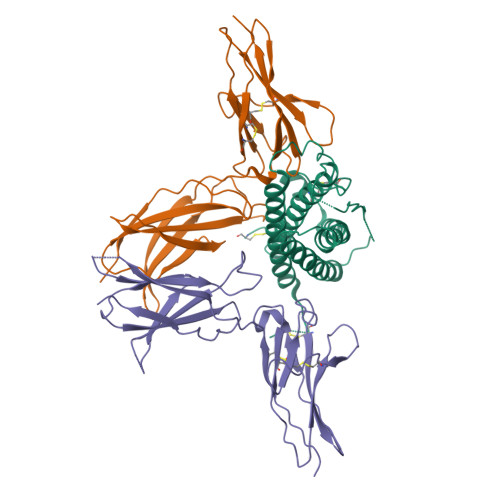
The most promising approach to targeting the tumor-growth-promoting actions of prolactin (PRL) mediated by its autocrine/paracrine pathway has been the development of specific PRL receptor (PRLR) antagonists. However, the optimization of such antagonists requires a thorough understanding of the activation mechanism of PRLR. We have thus conducted a systematic X-ray crystallographic study in order to visualize the successive steps of PRLR activation by PRL. We report here the structure at 3.35 Å resolution of the 1:2 complex between natural PRL and two PRLR chains (PRLR1 and PRLR2), corresponding to the final activated state of PRLR. Further than our previously published structure involving an affinity-matured PRL variant, this structure allowed to visualize for the first time the loop L5 spanning PRLR2 residues Thr133-Phe140, revealing its central implication for the three intermolecular interfaces of the complex. We equally succeeded in obtaining a comprehensive picture of the PRLR-PRLR dimerization interface, also called stem-stem interface. Site-directed mutagenesis was conducted to probe the energetic importance of stem-stem contacts highlighted by the structure. Surprisingly, in spite of significant structural differences between the PRL/PRLR(2) complex and the 1:2 growth hormone/growth hormone receptor complex, our mutational data suggest that hot-spot residues that stabilize the receptor dimerization interface are equivalent in the two complexes. This study provides a new overall picture of the structural features of PRLR involved in stabilizing its complex with PRL.
Organizational Affiliation:
CNRS UMR 8015, Laboratoire de cristallographie et RMN biologiques, F-75006 Paris, France.