Crystal structure of Plasmodium falciparum orotidine 5'-monophosphate decarboxylase complexed with 5-fluoro-6-amino-UMP, produced from 5-fluoro-6-azido-UMP
Liu, Y., Kotra, L.P., Pai, E.F.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Orotidine 5'-phosphate decarboxylase | 342 | Plasmodium falciparum 3D7 | Mutation(s): 0 Gene Names: gi|9310996, PF10_0225 EC: 4.1.1.23 | 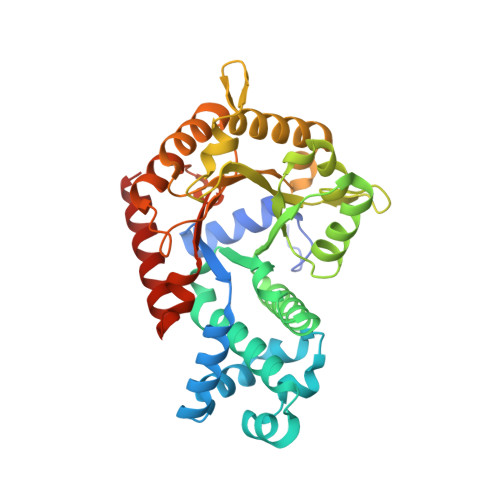 | |
UniProt | |||||
Find proteins for Q8IJH3 (Plasmodium falciparum (isolate 3D7)) Explore Q8IJH3 Go to UniProtKB: Q8IJH3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8IJH3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FNU Query on FNU | C [auth A], E [auth B] | 6-amino-5-fluorouridine 5'-(dihydrogen phosphate) C9 H13 F N3 O9 P OLBMCLUPWOAIRA-UMMCILCDSA-N |  | ||
| PGE Query on PGE | D [auth A] | TRIETHYLENE GLYCOL C6 H14 O4 ZIBGPFATKBEMQZ-UHFFFAOYSA-N |  | ||
| PEG Query on PEG | H [auth B] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| PO4 Query on PO4 | F [auth B] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| EDO Query on EDO | G [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 80.339 | α = 90 |
| b = 83.085 | β = 90 |
| c = 89.857 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| MOLREP | phasing |
| REFMAC | refinement |
| Coot | model building |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |