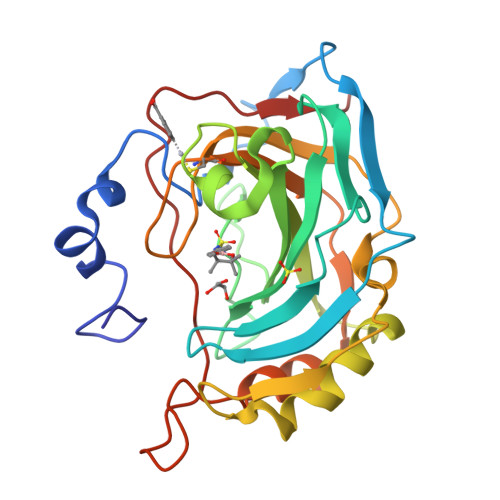
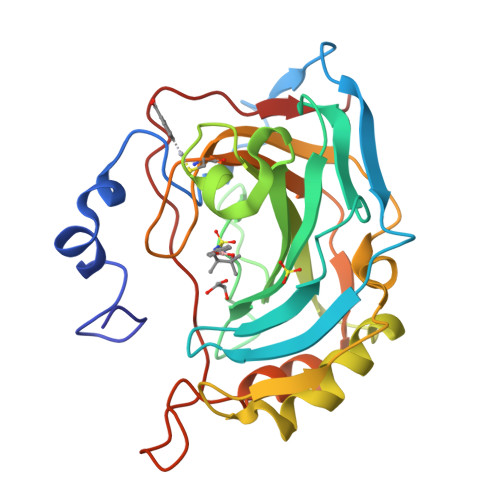
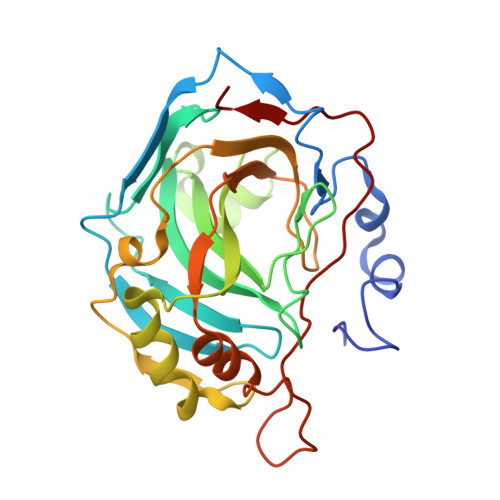
Carbonic anhydrase inhibitors: crystallographic and solution binding studies for the interaction of a boron-containing aromatic sulfamide with mammalian isoforms I-XV.
Di Fiore, A., Monti, S.M., Innocenti, A., Winum, J.Y., De Simone, G., Supuran, C.T.(2010) Bioorg Med Chem Lett 20: 3601-3605
- PubMed: 20472429
- DOI: https://doi.org/10.1016/j.bmcl.2010.04.114
- Primary Citation of Related Structures:
3MNU - PubMed Abstract:
We investigated the inhibition of carbonic anhydrase (CA, EC 4.2.1.1) isoforms I-XV with 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenylsulfamide and other simple or sugar sulfamides, a class of less investigated CA inhibitors (CAIs). The crystal structure of the adduct of hCA II with the boron-substituted sulfamide shows the organic scaffold of this compound bound in the hydrophilic half of the active site where it makes a large number of van der Waals contacts with Ile91, Gln92, Val121, Phe131, Leu198, and Thr200. The data here reported provide further insights into sulfamide binding mechanism confirming that this zinc-binding group could be usefully exploited for obtaining new potent and selective CAIs.
Organizational Affiliation:
Istituto di Biostrutture e Bioimmagini-CNR, Naples, Italy.