Kinetic, Mutational, and Structural Analysis of Malonate Semialdehyde Decarboxylase from Coryneform Bacterium Strain FG41: Mechanistic Implications for the Decarboxylase and Hydratase Activities.
Guo, Y., Serrano, H., Poelarends, G.J., Johnson, W.H., Hackert, M.L., Whitman, C.P.(2013) Biochemistry 52: 4830-4841
- PubMed: 23781927
- DOI: https://doi.org/10.1021/bi400567a
- Primary Citation of Related Structures:
3MJZ, 3MLC, 4LHO, 4LHP - PubMed Abstract:
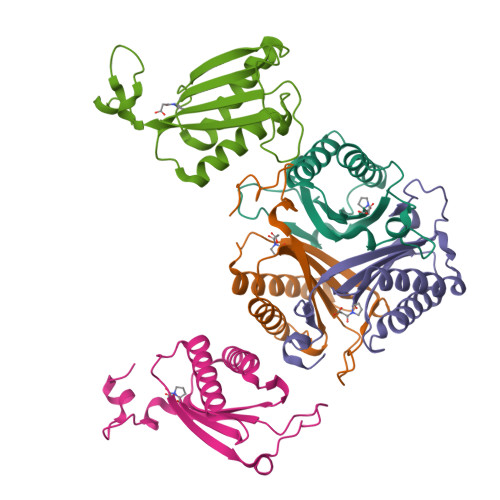
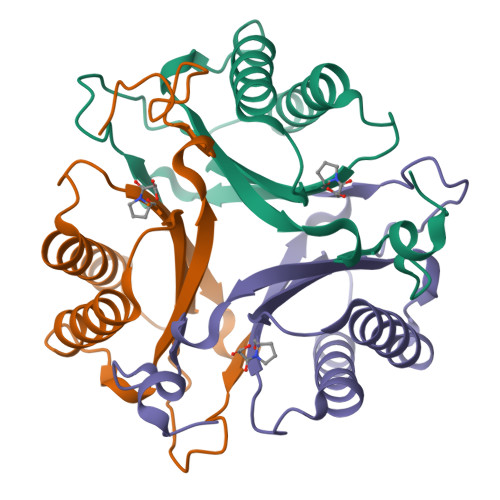
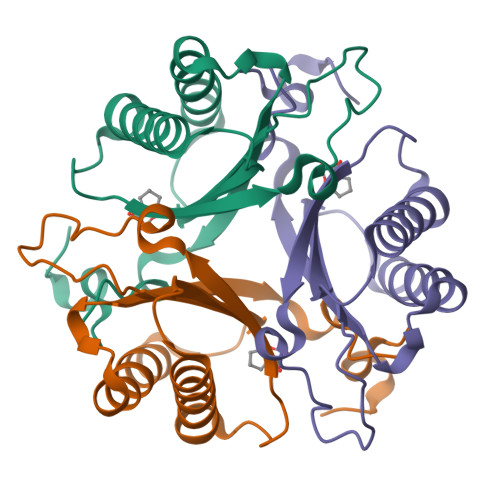
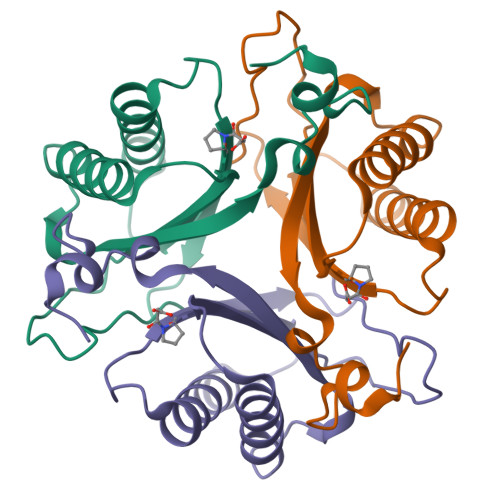
Malonate semialdehyde decarboxylase from Pseudomonas pavonaceae 170 (designated Pp MSAD) is in a bacterial catabolic pathway for the nematicide 1,3-dichloropropene. MSAD has two known activities: it catalyzes the metal ion-independent decarboxylation of malonate semialdehyde to produce acetaldehyde and carbon dioxide and a low-level hydration of 2-oxo-3-pentynoate to yield acetopyruvate. The latter activity is not known to be biologically relevant. Previous studies identified Pro-1, Asp-37, and a pair of arginines (Arg-73 and Arg-75) as critical residues in these activities. In terms of pairwise sequence, MSAD from Coryneform bacterium strain FG41 (designated FG41 MSAD) is 38% identical with the Pseudomonas enzyme, including Pro-1 and Asp-37. However, Gln-73 replaces Arg-73, and the second arginine is shifted to Arg-76 by the insertion of a glycine. To determine how these changes relate to the activities of FG41 MSAD, the gene was cloned and the enzyme expressed and characterized. The enzyme has a comparable decarboxylase activity but a significantly reduced hydratase activity. Mutagenesis along with crystal structures of the native enzyme (2.0 Å resolution) and the enzyme modified by a 3-oxopropanoate moiety (resulting from the incubation of the enzyme and 3-bromopropiolate) (2.2 Å resolution) provided a structural basis. The roles of Pro-1 and Asp-37 are likely the same as those proposed for Pp MSAD. However, the side chains of Thr-72, Gln-73, and Tyr-123 replace those of Arg-73 and Arg-75 in the mechanism and play a role in binding and catalysis. The structures also show that Arg-76 is likely too distant to play a direct role in the mechanism. FG41 MSAD is the second functionally annotated homologue in the MSAD family of the tautomerase superfamily and could represent a new subfamily.
Organizational Affiliation:
Division of Medicinal Chemistry, College of Pharmacy, and ‡Department of Chemistry and Biochemistry, The University of Texas , Austin, Texas 78712-1074, United States.