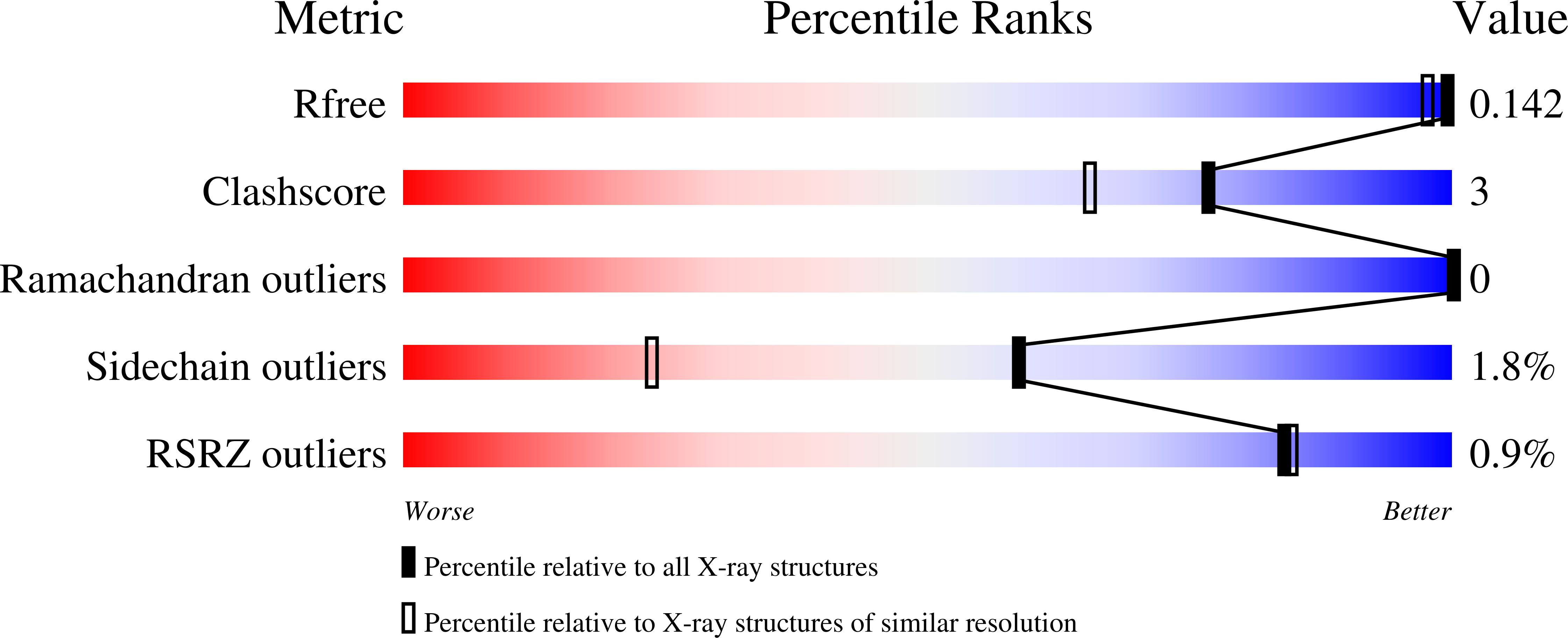
Ligand-induced fit affects binding modes and provokes changes in crystal packing of aldose reductase
Koch, C., Heine, A., Klebe, G.(2011) Biochim Biophys Acta 1810: 879-887
- PubMed: 21684320
- DOI: https://doi.org/10.1016/j.bbagen.2011.06.001
- Primary Citation of Related Structures:
3LEN, 3M0I, 3M64, 3MB9, 3MC5, 3P2V - PubMed Abstract:
Flexibility is a common feature of proteins. For human aldose reductase, a variety of conformers have been observed in crystalline complexes with different inhibitors. A study of crystal structures and isothermal titration calorimetry was performed on wild type and mutated aldose reductase. Though the interaction to the mutated residue Thr113 does not directly alter the binding mode of zopolrestat to aldose reductase, a shift of its basic scaffold is induced which affects the interaction with a flexible loop and introduces disorder. With the related inhibitor IDD393, two distinct binding site conformations result in two different crystal forms: While a backbone flip of the same residues as for zopolrestat is present in both crystal forms, a considerable side-chain movement of a phenylalanine is observed for only one crystal form. In consequence, residual mobility of adjacent amino acids is increased and some crystal contacts are prevented which reinforces different crystal packing. The structure of a benzothiazepine reveals a protein conformer, where this phenylalanine is further relocated resulting in the same altered crystal packing. Differences in the thermodynamic signature recorded for the various complexes relate to the structural differences. Crystal structures are accepted as "gold standard" for the interpretation of protein geometry, however, they are only one possible structure and can be influenced by crystal packing. In reverse, ligand binding can affect protein conformation and determine crystal packing. The phenomenon of such "polymorphic forms" is well appreciated, however rarely understood at the molecular level.
Organizational Affiliation:
Philipps-Universität, Department of Pharmaceutical Chemistry, Marburg, Germany.