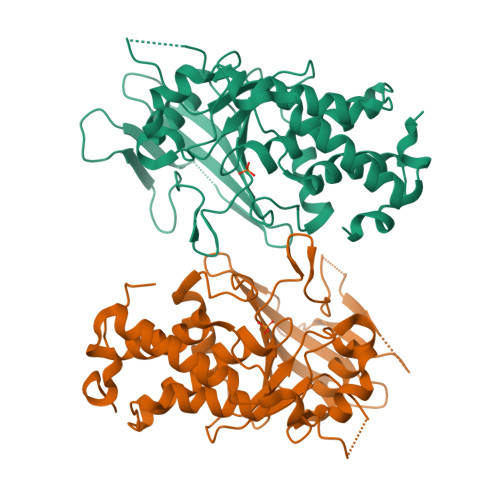
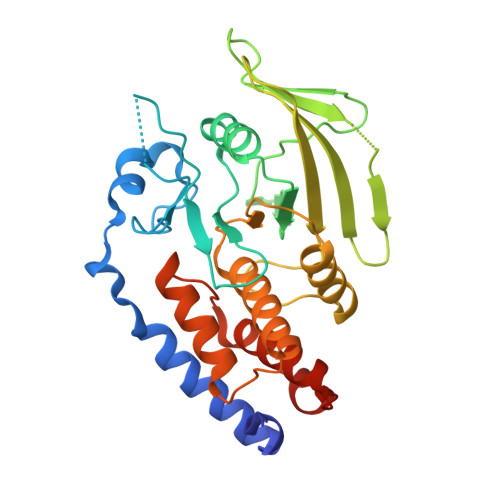
The Trypanosoma brucei life cycle switch TbPTP1 is structurally conserved and dephosphorylates the nucleolar protein NOPP44/46.
Chou, S., Jensen, B.C., Parsons, M., Alber, T., Grundner, C.(2010) J Biological Chem 285: 22075-22081
- PubMed: 20444707
- DOI: https://doi.org/10.1074/jbc.M110.108860
- Primary Citation of Related Structures:
3M4U - PubMed Abstract:
Trypanosoma brucei adapts to changing environments as it cycles through arrested and proliferating stages in the human and tsetse fly hosts. Changes in protein tyrosine phosphorylation of several proteins, including NOPP44/46, accompany T. brucei development. Moreover, inactivation of T. brucei protein-tyrosine phosphatase 1 (TbPTP1) triggers differentiation of bloodstream stumpy forms into tsetse procyclic forms through unknown downstream effects. Here, we link these events by showing that NOPP44/46 is a major substrate of TbPTP1. TbPTP1 substrate-trapping mutants selectively enrich NOPP44/46 from procyclic stage cell lysates, and TbPTP1 efficiently and selectively dephosphorylates NOPP44/46 in vitro. To provide insights into the mechanism of NOPP44/46 recognition, we determined the crystal structure of TbPTP1. The TbPTP1 structure, the first of a kinetoplastid protein-tyrosine phosphatase (PTP), emphasizes the conservation of the PTP fold, extending to one of the most diverged eukaryotes. The structure reveals surfaces that may mediate substrate specificity and affords a template for the design of selective inhibitors to interfere with T. brucei transmission.
Organizational Affiliation:
Department of Molecular and Cell Biology and QB3 Institute, University of California, Berkeley, California 94720-3200, USA.