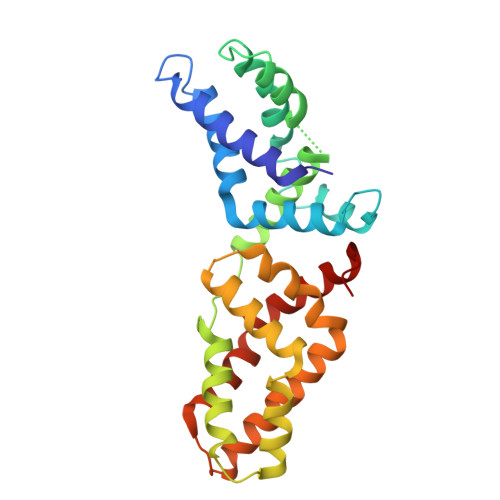
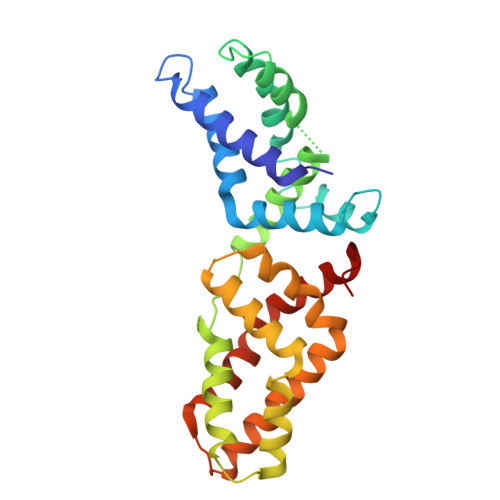
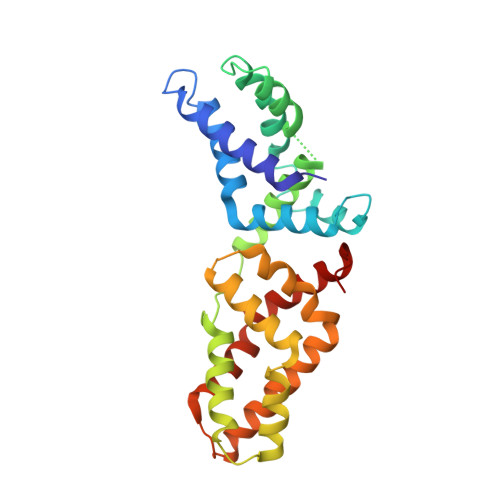
Structural basis for tandem L27 domain-mediated polymerization
Yang, X., Xie, X., Chen, L., Zhou, H., Wang, Z., Zhao, W., Tian, R., Zhang, R., Tian, C., Long, J., Shen, Y.(2010) FASEB J 24: 4806-4815
- PubMed: 20702775
- DOI: https://doi.org/10.1096/fj.10-163857
- Primary Citation of Related Structures:
3LRA - PubMed Abstract:
The establishment of epithelial cell polarity requires the assembly of multiprotein complexes and is crucial during epithelial morphogenesis. Three scaffolding proteins, Dlg1, MPP7, and Mals3, can be assembled to form a complex that functions in the establishment and maintenance of apicobasal polarity in epithelial tissues through their L27 domains. Here we report the crystal structure of a 4-L27-domain complex derived from the human tripartite complex Dlg1-MPP7-Mals3 in combination with paramagnetic relaxation enhancement measurements. The heterotrimer consists of 2 pairs of heterodimeric L27 domains. These 2 dimers are asymmetric due to the large difference between the N- and C-terminal tandem L27 domain of MPP7. Structural analysis combined with biochemical experiments further reveals that the loop αA-αB and helix αB of the C-terminal L27 domain of MPP7 play a critical role in assembling the entire tripartite complex, suggesting a synergistic tandem L27-mediated assembling event.
Organizational Affiliation:
Tianjin Key Laboratory of Protein Science, College of Life Science, Nankai University, Tianjin, China.