Discovery and SAR of potent, orally available 2,8-diaryl-quinoxalines as a new class of JAK2 inhibitors
Pissot-Soldermann, C., Gerspacher, M., Furet, P., Gaul, C., Holzer, P., McCarthy, C., Radimerski, T., Regnier, C.H., Baffert, F., Drueckes, P., Tavares, G.A., Vangrevelinghe, E., Blasco, F., Ottaviani, G., Ossola, F., Scesa, J., Reetz, J.(2010) Bioorg Med Chem Lett 20: 2609-2613
- PubMed: 20231096
- DOI: https://doi.org/10.1016/j.bmcl.2010.02.056
- Primary Citation of Related Structures:
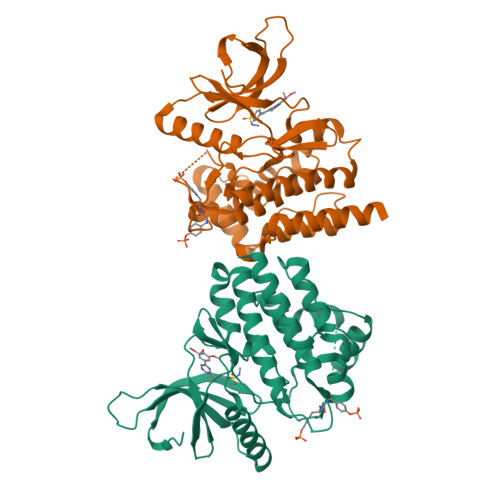
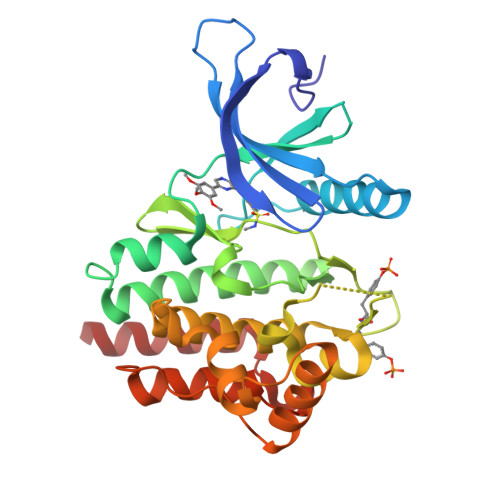
3LPB - PubMed Abstract:
We have designed and synthesized a novel series of 2,8-diaryl-quinoxalines as Janus kinase 2 inhibitors. Many of the inhibitors show low nanomolar activity against JAK2 and potently suppress proliferation of SET-2 cells in vitro. In addition, compounds from this series have favorable rat pharmacokinetic properties suitable for in vivo efficacy evaluation.
Organizational Affiliation:
Novartis Institutes for Biomedical Research, Novartis Pharma AG, WKL-136.4.96, CH-4002 Basel, Switzerland. carole.pissot@novartis.com