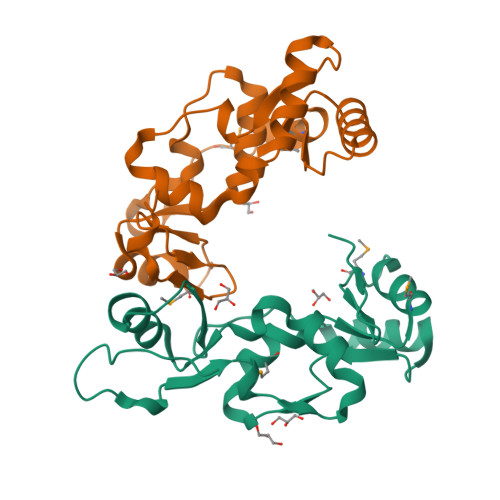
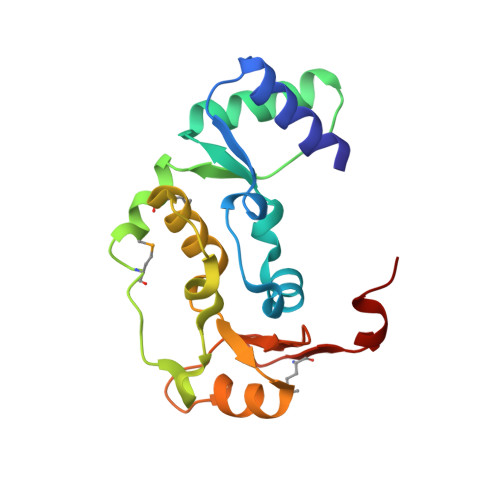
Structure and DNA binding activity of the mouse condensin hinge domain highlight common and diverse features of SMC proteins
Griese, J.J., Witte, G., Hopfner, K.-P.(2010) Nucleic Acids Res 38: 3454-3465
- PubMed: 20139420
- DOI: https://doi.org/10.1093/nar/gkq038
- Primary Citation of Related Structures:
3L51 - PubMed Abstract:
Structural Maintenance of Chromosomes (SMC) proteins are vital for a wide range of processes including chromosome structure and dynamics, gene regulation and DNA repair. Eukaryotes have three SMC complexes, consisting of heterodimeric pairs of six different SMC proteins along with several specific regulatory subunits. In addition to their other functions, all three SMC complexes play distinct roles in DNA repair. Cohesin (SMC1-SMC3) is involved in DNA double-strand break repair, condensin (SMC2-SMC4) participates in single-strand break (SSB) repair, and the SMC5-SMC6 complex functions in various DNA repair pathways. SMC proteins consist of N- and C-terminal domains that fold back onto each other to create an ATPase 'head' domain, connected to a central 'hinge' domain via long coiled-coils. The hinge domain mediates dimerization of SMC proteins and binds DNA, but it is not clear to what purpose this activity serves. We studied the structure and function of the condensin hinge domain from mouse. While the SMC hinge domain structure is largely conserved from prokaryotes to eukaryotes, its function seems to have diversified throughout the course of evolution. The condensin hinge domain preferentially binds single-stranded DNA. We propose that this activity plays a role in the SSB repair function of the condensin complex.
Organizational Affiliation:
Department of Biochemistry, Gene Center, Center for Integrated Protein Sciences and Munich Center for Advanced Photonics, Ludwig-Maximilians University Munich, Feodor-Lynen-Str 25, D-81377 Munich, Germany.