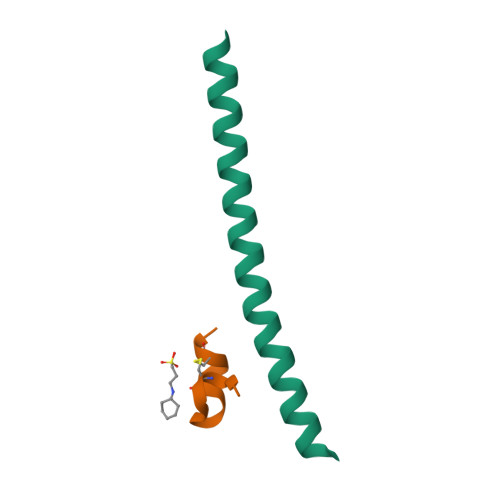
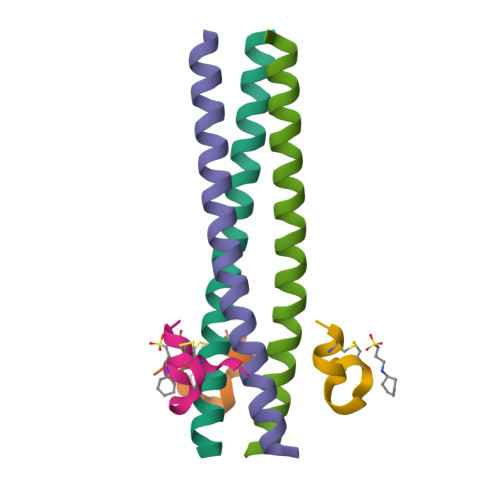
Design of a potent D-peptide HIV-1 entry inhibitor with a strong barrier to resistance.
Welch, B.D., Francis, J.N., Redman, J.S., Paul, S., Weinstock, M.T., Reeves, J.D., Lie, Y.S., Whitby, F.G., Eckert, D.M., Hill, C.P., Root, M.J., Kay, M.S.(2010) J Virol 84: 11235-11244
- PubMed: 20719956
- DOI: https://doi.org/10.1128/JVI.01339-10
- Primary Citation of Related Structures:
3L35, 3L36, 3L37, 3MGN - PubMed Abstract:
The HIV gp41 N-trimer pocket region is an ideal viral target because it is extracellular, highly conserved, and essential for viral entry. Here, we report on the design of a pocket-specific D-peptide, PIE12-trimer, that is extraordinarily elusive to resistance and characterize its inhibitory and structural properties. D-peptides (peptides composed of D-amino acids) are promising therapeutic agents due to their insensitivity to protease degradation. PIE12-trimer was designed using structure-guided mirror-image phage display and linker optimization and is the first D-peptide HIV entry inhibitor with the breadth and potency required for clinical use. PIE12-trimer has an ultrahigh affinity for the gp41 pocket, providing it with a reserve of binding energy (resistance capacitor) that yields a dramatically improved resistance profile compared to those of other fusion inhibitors. These results demonstrate that the gp41 pocket is an ideal drug target and establish PIE12-trimer as a leading anti-HIV antiviral candidate.
Organizational Affiliation:
Department of Biochemistry, University of Utah School of Medicine, 15 N. Medical Drive East, Rm. 4100, Salt Lake City, UT 84112-5650, USA.