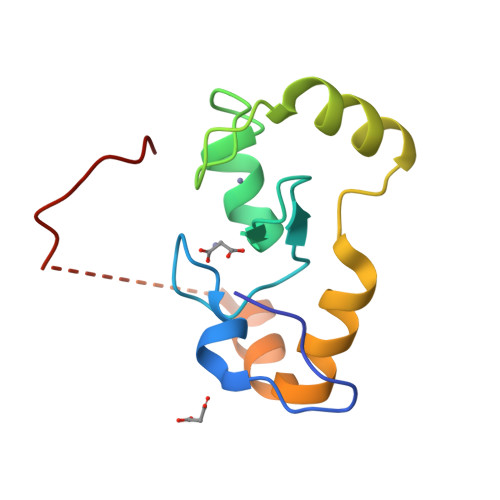
Molecular insights into the function of RING finger (RNF)-containing proteins hRNF8 and hRNF168 in Ubc13/Mms2-dependent ubiquitylation.
Campbell, S.J., Edwards, R.A., Leung, C.C., Neculai, D., Hodge, C.D., Dhe-Paganon, S., Glover, J.N.(2012) J Biological Chem 287: 23900-23910
- PubMed: 22589545
- DOI: https://doi.org/10.1074/jbc.M112.359653
- Primary Citation of Related Structures:
3L11, 4ORH - PubMed Abstract:
The repair of DNA double strand breaks by homologous recombination relies on the unique topology of the chains formed by Lys-63 ubiquitylation of chromatin to recruit repair factors such as breast cancer 1 (BRCA1) to sites of DNA damage. The human RING finger (RNF) E3 ubiquitin ligases, RNF8 and RNF168, with the E2 ubiquitin-conjugating complex Ubc13/Mms2, perform the majority of Lys-63 ubiquitylation in homologous recombination. Here, we show that RNF8 dimerizes and binds to Ubc13/Mms2, thereby stimulating formation of Lys-63 ubiquitin chains, whereas the related RNF168 RING domain is a monomer and does not catalyze Lys-63 polyubiquitylation. The crystal structure of the RNF8/Ubc13/Mms2 ternary complex reveals the structural basis for the interaction between Ubc13 and the RNF8 RING and that an extended RNF8 coiled-coil is responsible for its dimerization. Mutations that disrupt the RNF8/Ubc13 binding surfaces, or that truncate the RNF8 coiled-coil, reduce RNF8-catalyzed ubiquitylation. These findings support the hypothesis that RNF8 is responsible for the initiation of Lys-63-linked ubiquitylation in the DNA damage response, which is subsequently amplified by RNF168.
Organizational Affiliation:
Department of Biochemistry, University of Alberta, Edmonton, Alberta, Canada T6G 2H7.