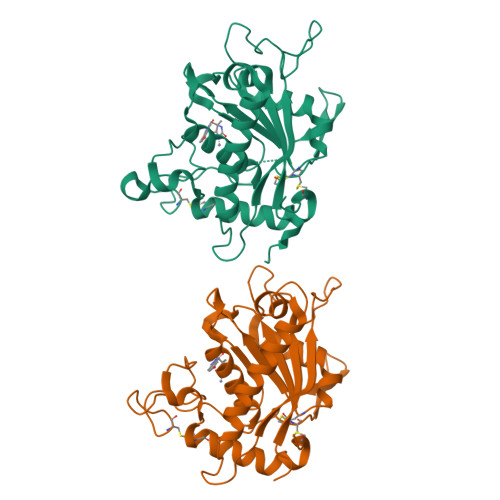
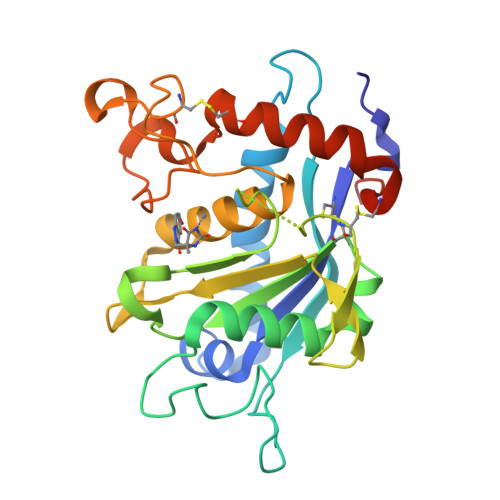
Discovery and SAR of hydantoin TACE inhibitors.
Yu, W., Guo, Z., Orth, P., Madison, V., Chen, L., Dai, C., Feltz, R.J., Girijavallabhan, V.M., Kim, S.H., Kozlowski, J.A., Lavey, B.J., Li, D., Lundell, D., Niu, X., Piwinski, J.J., Popovici-Muller, J., Rizvi, R., Rosner, K.E., Shankar, B.B., Shih, N.Y., Siddiqui, M.A., Sun, J., Tong, L., Umland, S., Wong, M.K., Yang, D.Y., Zhou, G.(2010) Bioorg Med Chem Lett 20: 1877-1880
- PubMed: 20172725
- DOI: https://doi.org/10.1016/j.bmcl.2010.01.148
- Primary Citation of Related Structures:
3L0T, 3L0V - PubMed Abstract:
We disclose inhibitors of TNF-alpha converting enzyme (TACE) designed around a hydantoin zinc binding moiety. Crystal structures of inhibitors bound to TACE revealed monodentate coordination of the hydantoin to the zinc. SAR, X-ray, and modeling designs are described. To our knowledge, these are the first reported X-ray structures of TACE with a hydantoin zinc ligand.
Organizational Affiliation:
Department of Medicinal Chemistry, Merck Research Laboratories, Kenilworth, NJ 07033, USA. wensheng.yu@spcorp.com