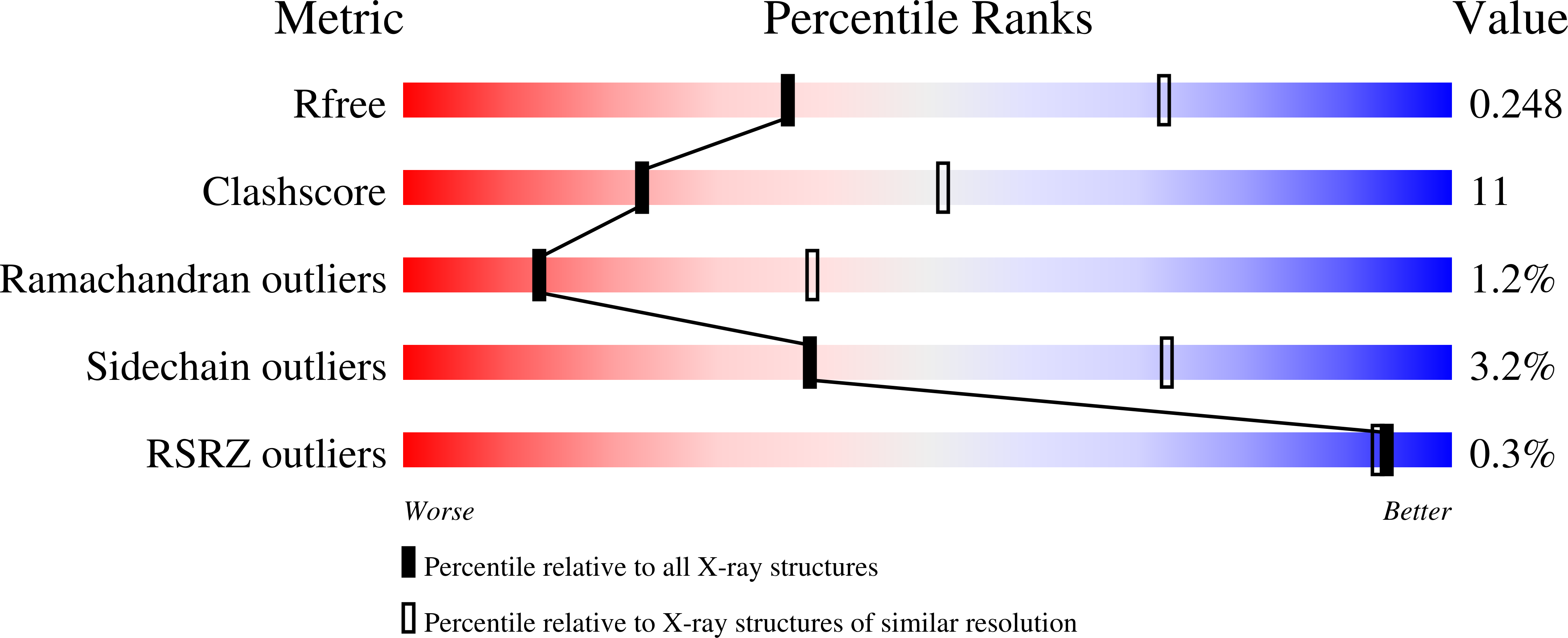
A single mutation in the active site swaps the substrate specificity of N-acetyl-L-ornithine transcarbamylase and N-succinyl-L-ornithine transcarbamylase.
Shi, D., Yu, X., Cabrera-Luque, J., Chen, T.Y., Roth, L., Morizono, H., Allewell, N.M., Tuchman, M.(2007) Protein Sci 16: 1689-1699
- PubMed: 17600144
- DOI: https://doi.org/10.1110/ps.072919907
- Primary Citation of Related Structures:
2G7M, 3L02, 3L04, 3L05, 3L06 - PubMed Abstract:
Transcarbamylases catalyze the transfer of the carbamyl group from carbamyl phosphate (CP) to an amino group of a second substrate such as aspartate, ornithine, or putrescine. Previously, structural determination of a transcarbamylase from Xanthomonas campestris led to the discovery of a novel N-acetylornithine transcarbamylase (AOTCase) that catalyzes the carbamylation of N-acetylornithine. Recently, a novel N-succinylornithine transcarbamylase (SOTCase) from Bacteroides fragilis was identified. Structural comparisons of AOTCase from X. campestris and SOTCase from B. fragilis revealed that residue Glu92 (X. campestris numbering) plays a critical role in distinguishing AOTCase from SOTCase. Enzymatic assays of E92P, E92S, E92V, and E92A mutants of AOTCase demonstrate that each of these mutations converts the AOTCase to an SOTCase. Similarly, the P90E mutation in B. fragilis SOTCase (equivalent to E92 in X. campestris AOTCase) converts the SOTCase to AOTCase. Hence, a single amino acid substitution is sufficient to swap the substrate specificities of AOTCase and SOTCase. X-ray crystal structures of these mutants in complexes with CP and N-acetyl-L-norvaline (an analog of N-acetyl-L-ornithine) or N-succinyl-L-norvaline (an analog of N-succinyl-L-ornithine) substantiate this conversion. In addition to Glu92 (X. campestris numbering), other residues such as Asn185 and Lys30 in AOTCase, which are involved in binding substrates through bridging water molecules, help to define the substrate specificity of AOTCase. These results provide the correct annotation (AOTCase or SOTCase) for a set of the transcarbamylase-like proteins that have been erroneously annotated as ornithine transcarbamylase (OTCase, EC 2.1.3.3).
Organizational Affiliation:
Children's National Medical Center, The George Washington University, Washington, DC 20010, USA. dshi@cnmcresearch.org