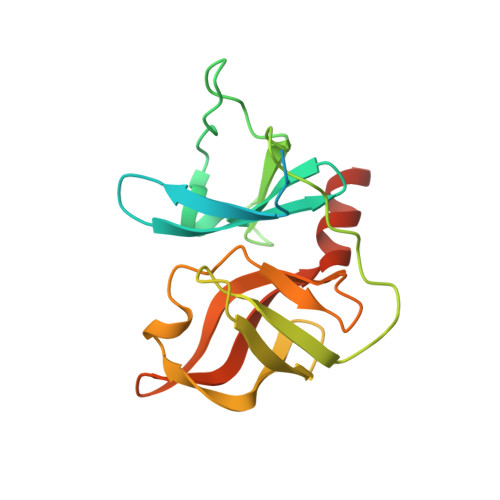
Induced-Fit Binding of the Macrocyclic Noncovalent Inhibitor TMC435 to its HCV NS3/NS4A Protease Target
Cummings, M.D., Lindberg, J.D., Lin, T.-I., de Kock, H., Lenz, O., Lilja, E., Fellander, S., Baraznenok, V., Nystrom, S., Nilsson, M., Vrang, L., Edlund, M., Rosenquist, A., Samuelsson, B., Raboisson, P., Simmen, K.(2010) Angew Chem Int Ed Engl 49: 1652-1655
- PubMed: 20166108
- DOI: https://doi.org/10.1002/anie.200906696
- Primary Citation of Related Structures:
3KEE, 3KF2
Organizational Affiliation:
Tibotec BVBA, Gen De Wittelaan L 11B 3, 2800 Mechelen, Belgium. mcummin1@its.jnj.com