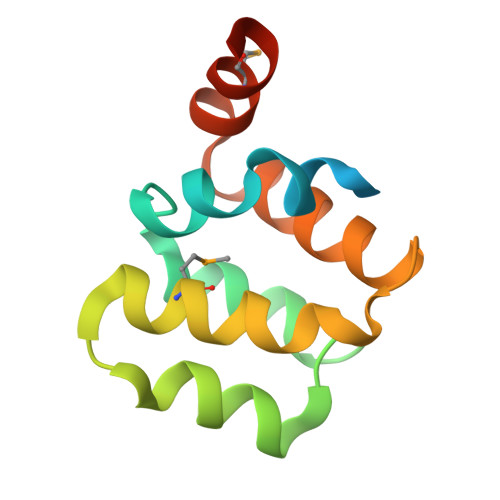
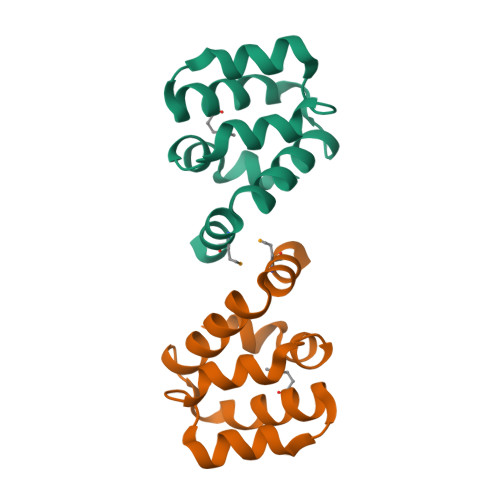
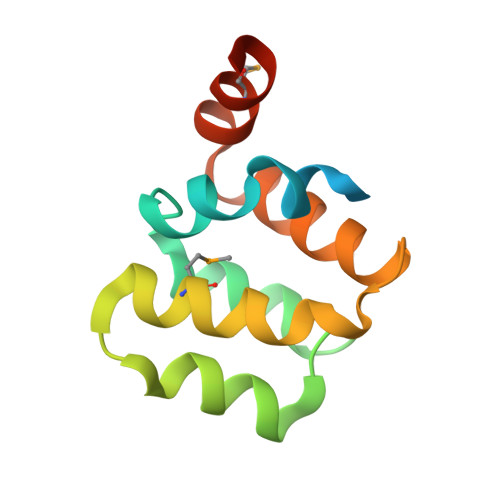
Northeast Structural Genomics Consortium Target HR3486E
Forouhar, F., Abashidze, M., Seetharaman, J., Mao, M., Xiao, R., Ciccosanti, C., Shastry, R., Everett, J.K., Nair, R., Acton, T.B., Rost, B., Montelione, G.T., Tong, L., Hunt, J.F.To be published.