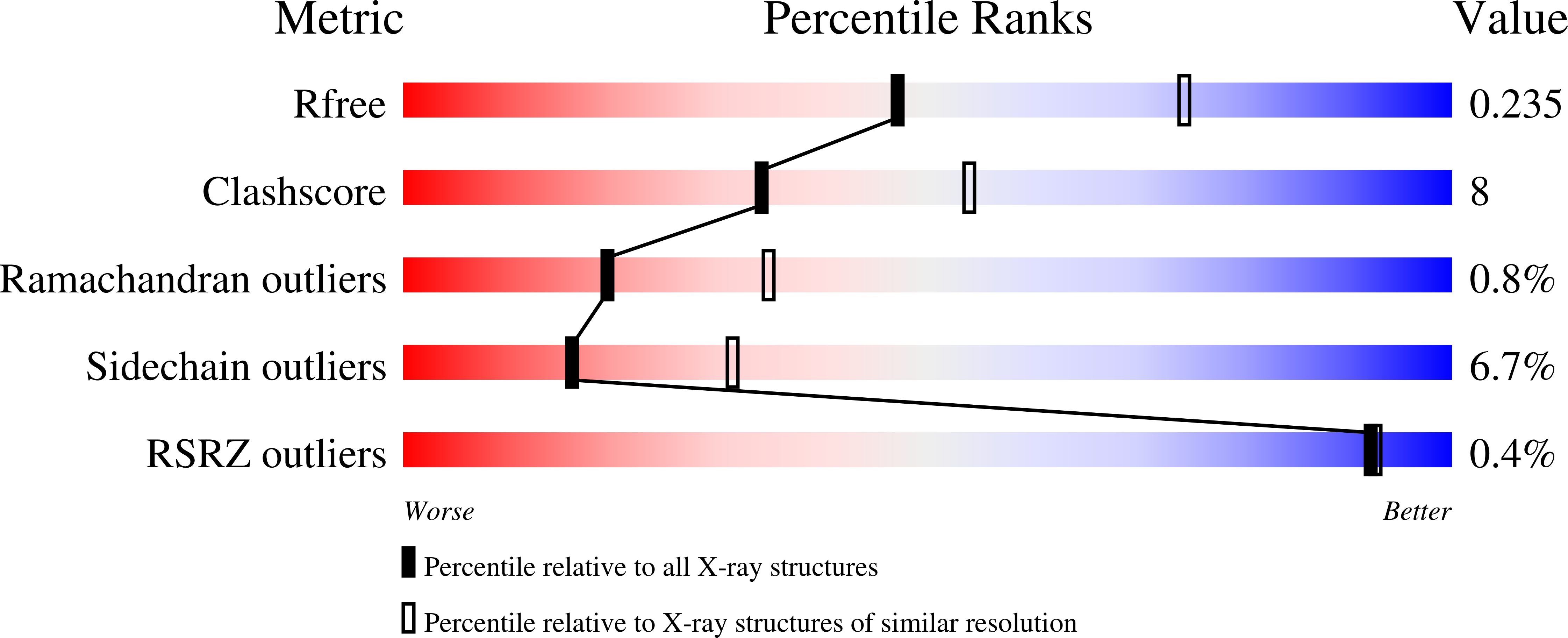
Structure-based mechanism of CMP-2-keto-3-deoxymanno-octulonic acid synthetase: convergent evolution of a sugar-activating enzyme with DNA/RNA polymerases
Heyes, D.J., Levy, C., Lafite, P., Roberts, I.S., Goldrick, M., Stachulski, A.V., Rossington, S.B., Stanford, D., Rigby, S.E.J., Scrutton, N.S., Leys, D.(2009) J Biol Chem 284: 35514-35523
- PubMed: 19815542
- DOI: https://doi.org/10.1074/jbc.M109.056630
- Primary Citation of Related Structures:
3K8D, 3K8E - PubMed Abstract:
The enzyme CMP-Kdo synthetase (KdsB) catalyzes the addition of 2-keto-3-deoxymanno-octulonic acid (Kdo) to CTP to form CMP-Kdo, a key reaction in the biosynthesis of lipopolysaccharide. The reaction catalyzed by KdsB and the related CMP-acylneuraminate synthase is unique among the sugar-activating enzymes in that the respective sugars are directly coupled to a cytosine monophosphate. Using inhibition studies, in combination with isothermal calorimetry, we show the substrate analogue 2beta-deoxy-Kdo to be a potent competitive inhibitor. The ligand-free Escherichia coli KdsB and ternary complex KdsB-CTP-2beta-deoxy-Kdo crystal structures reveal that Kdo binding leads to active site closure and repositioning of the CTP phosphates and associated Mg(2+) ion (Mg-B). Both ligands occupy conformations compatible with an S(n)2-type attack on the alpha-phosphate by the Kdo 2-hydroxyl group. Based on strong similarity with DNA/RNA polymerases, both in terms of overall chemistry catalyzed as well as active site configuration, we postulate a second Mg(2+) ion (Mg-A) is bound by the catalytically competent KdsB-CTP-Kdo ternary complex. Modeling of this complex reveals the Mg-A coordinated to the conserved Asp(100) and Asp(235) in addition to the CTP alpha-phosphate and both the Kdo carboxylic and 2-hydroxyl groups. EPR measurements on the Mn(2+)-substituted ternary complex support this model. We propose the KdsB/CNS sugar-activating enzymes catalyze the formation of activated sugars, such as the abundant CMP-5-N-acetylneuraminic acid, by recruitment of two Mg(2+) to the active site. Although each metal ion assists in correct positioning of the substrates and activation of the alpha-phosphate, Mg-A is responsible for activation of the sugar-hydroxyl group.
Organizational Affiliation:
Manchester Interdisciplinary Biocentre, Faculty of Life Sciences, University of Manchester, 131 Princess Street, Manchester M1 7DN.