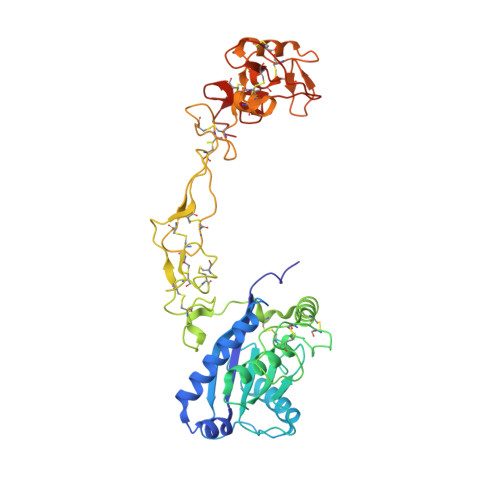
Structures of two elapid snake venom metalloproteases with distinct activities highlight the disulfide patterns in the D domain of ADAMalysin family proteins
Guan, H.H., Goh, K.S., Davamani, F., Wu, P.L., Huang, Y.W., Jeyakanthan, J., Wu, W.G., Chen, C.J.(2010) J Struct Biol 169: 294-303
- PubMed: 19932752
- DOI: https://doi.org/10.1016/j.jsb.2009.11.009
- Primary Citation of Related Structures:
3K7L, 3K7N - PubMed Abstract:
The structures of snake venom metalloproteases (SVMPs) are proposed to be useful models to understand the structural and functional relationship of ADAM (a disintegrin and metalloprotease) which are membrane-anchored proteins involved in multiple human diseases. We have purified, sequenced and determined the structures of two new P-III SVMPs - atragin and kaouthiagin-like (K-like) from Naja atra. Atragin exhibits a known C-shaped topology, whereas K-like adopts an I-shaped conformation because of the distinct disulfide pattern in the disintegrin-like (D) domain. K-like exhibits an enzymatic specificity toward pro-TNFalpha with less inhibition of cell migration, but atragin shows the opposite effect. The specificity of the enzymatic activity is indicated to be dominated mainly by the local structures of SVMP in the metalloprotease (M) domain, whereas the hyper-variable region (HVR) in the cysteine-rich (C) domain is involved in a cell-migration activity. We demonstrate also a pH-dependent enzymatic activity of atragin that we correlate with the structural dynamics of a Zn(2+)-binding motif and the Met-turn based on the structures determined with a pH-jump method. The structural variations between the C- and I-shapes highlight the disulfide bond patterns in the D domain of the ADAM/adamalysin/reprolysins family proteins.
Organizational Affiliation:
Life Science Group, Scientific Research Division, National Synchrotron Radiation Research Center, Hsinchu 30076, Taiwan.