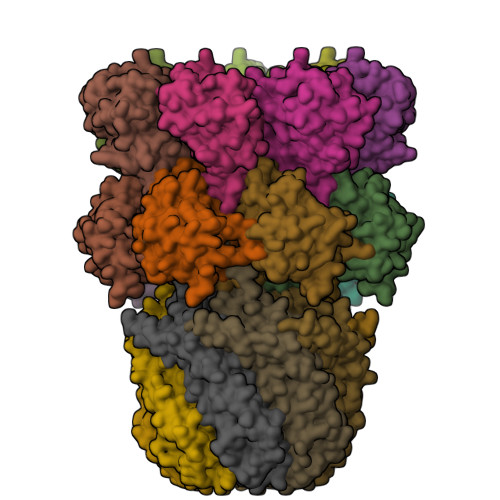
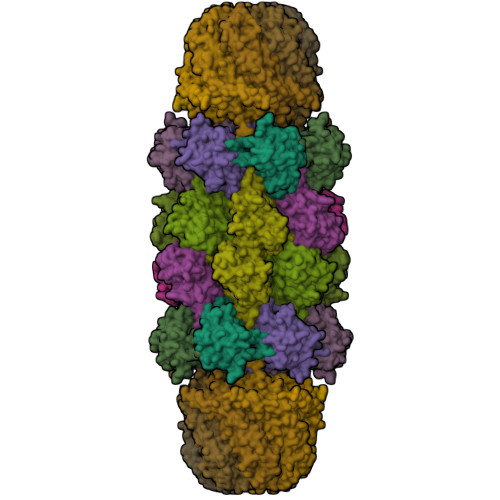
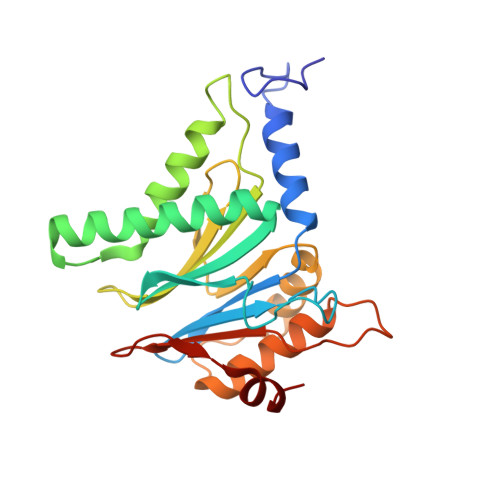
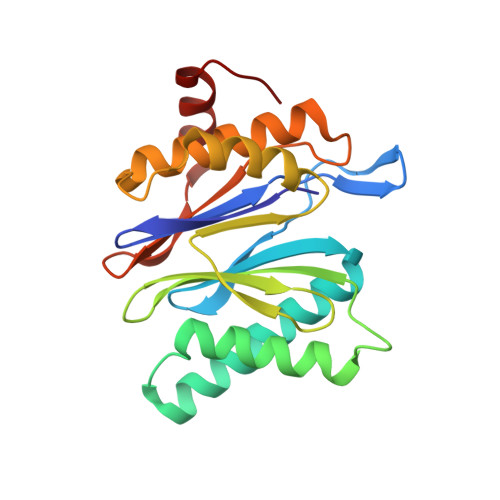
Structural models for interactions between the 20S proteasome and its PAN/19S activators.
Stadtmueller, B.M., Ferrell, K., Whitby, F.G., Heroux, A., Robinson, H., Myszka, D.G., Hill, C.P.(2010) J Biological Chem 285: 13-17
- PubMed: 19889631
- DOI: https://doi.org/10.1074/jbc.C109.070425
- Primary Citation of Related Structures:
3JRM, 3JSE, 3JTL - PubMed Abstract:
Proteasome activity is regulated by sequestration of its proteolytic centers in a barrel-shaped structure that limits substrate access. Substrates enter the proteasome by means of activator complexes that bind to the end rings of proteasome alpha subunits and induce opening of an axial entrance/exit pore. The PA26 activator binds in a pocket on the proteasome surface using main chain contacts of its C-terminal residues and uses an internal activation loop to trigger gate opening by repositioning the proteasome Pro-17 reverse turn. Subunits of the unrelated PAN/19S activators bind with their C termini in the same pockets but can induce proteasome gate opening entirely from interactions of their C-terminal peptides, which are reported to cause gate opening by inducing a rocking motion of proteasome alpha subunits rather than by directly contacting the Pro-17 turn. Here we report crystal structures and binding studies of proteasome complexes with PA26 constructs that display modified C-terminal residues, including those corresponding to PAN. These findings suggest that PA26 and PAN/19S C-terminal residues bind superimposably and that both classes of activator induce gate opening by using direct contacts to residues of the proteasome Pro-17 reverse turn. In the case of the PAN and 19S activators, a penultimate tyrosine/phenylalanine residue contacts the proteasome Gly-19 carbonyl oxygen to stabilize the open conformation.
Organizational Affiliation:
Department of Biochemistry, University of Utah School of Medicine, Salt Lake City, Utah 84112, USA.