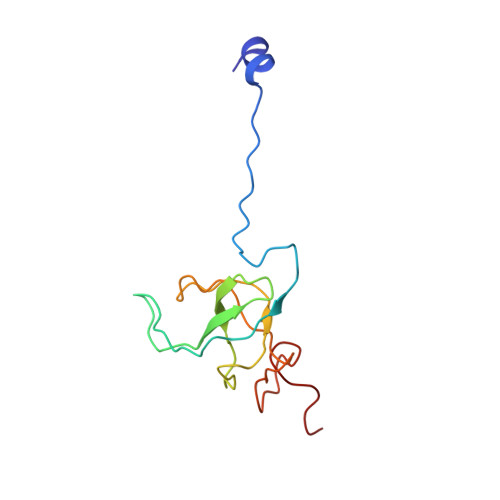
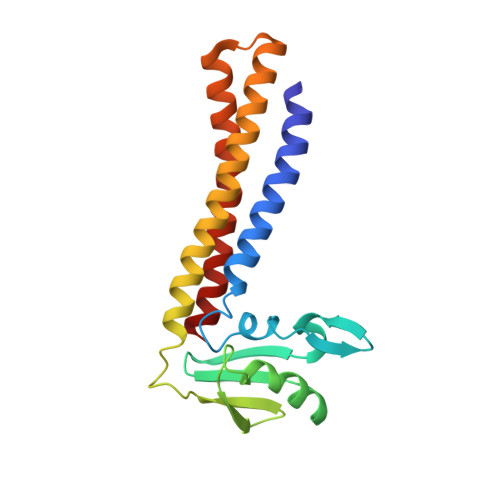
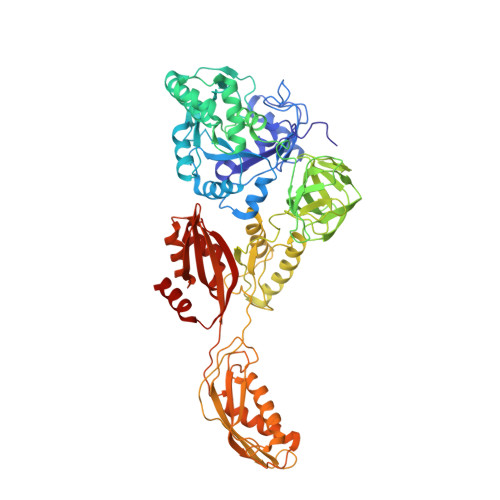
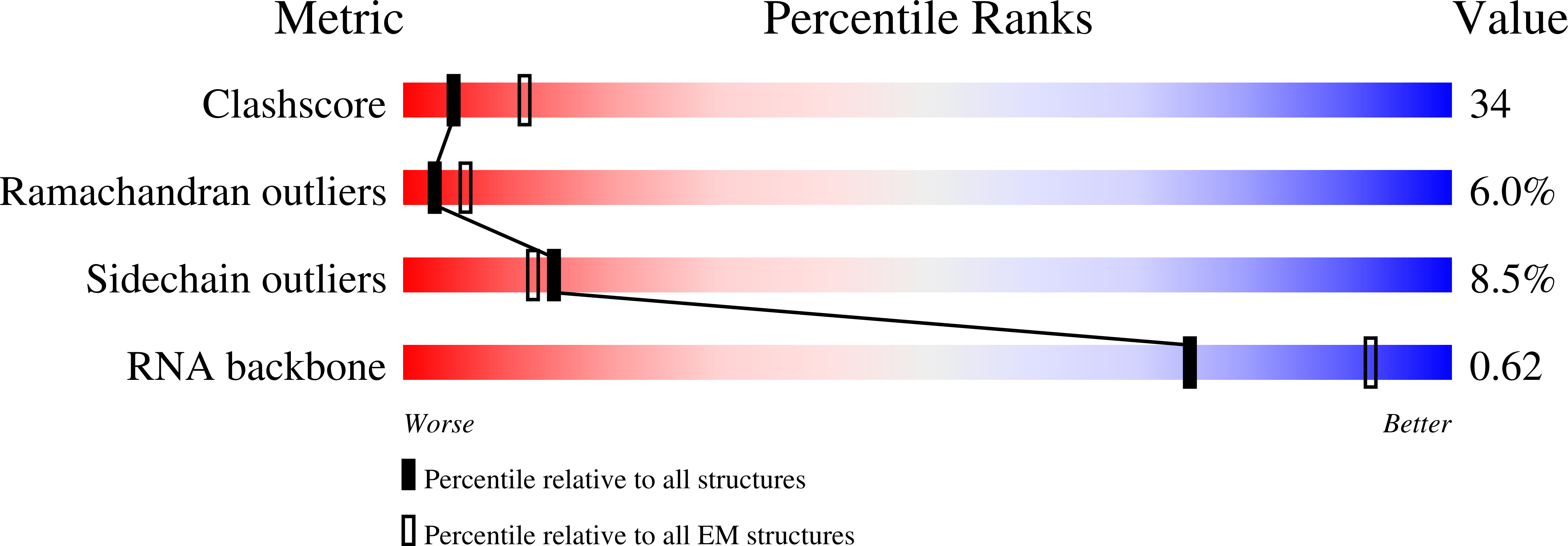Structural insights into initial and intermediate steps of the ribosome-recycling process.
Yokoyama, T., Shaikh, T.R., Iwakura, N., Kaji, H., Kaji, A., Agrawal, R.K.(2012) EMBO J 31: 1836-1846
- PubMed: 22388519
- DOI: https://doi.org/10.1038/emboj.2012.22
- Primary Citation of Related Structures:
3J0D, 3J0E - PubMed Abstract:
The ribosome-recycling factor (RRF) and elongation factor-G (EF-G) disassemble the 70S post-termination complex (PoTC) into mRNA, tRNA, and two ribosomal subunits. We have determined cryo-electron microscopic structures of the PoTC·RRF complex, with and without EF-G. We find that domain II of RRF initially interacts with universally conserved residues of the 23S rRNA helices 43 and 95, and protein L11 within the 50S ribosomal subunit. Upon EF-G binding, both RRF and tRNA are driven towards the tRNA-exit (E) site, with a large rotational movement of domain II of RRF towards the 30S ribosomal subunit. During this intermediate step of the recycling process, domain II of RRF and domain IV of EF-G adopt hitherto unknown conformations. Furthermore, binding of EF-G to the PoTC·RRF complex reverts the ribosome from ratcheted to unratcheted state. These results suggest that (i) the ribosomal intersubunit reorganizations upon RRF binding and subsequent EF-G binding could be instrumental in destabilizing the PoTC and (ii) the modes of action of EF-G during tRNA translocation and ribosome-recycling steps are markedly different.
Organizational Affiliation:
Division of Translational Medicine, Wadsworth Center, New York State Department of Health, Albany, NY, USA.