Lead optimization and structure-based design of potent and bioavailable deoxycytidine kinase inhibitors.
Jessop, T.C., Tarver, J.E., Carlsen, M., Xu, A., Healy, J.P., Heim-Riether, A., Fu, Q., Taylor, J.A., Augeri, D.J., Shen, M., Stouch, T.R., Swanson, R.V., Tari, L.W., Hunter, M., Hoffman, I., Keyes, P.E., Yu, X.C., Miranda, M., Liu, Q., Swaffield, J.C., David Kimball, S., Nouraldeen, A., Wilson, A.G., Foushee, A.M., Jhaver, K., Finch, R., Anderson, S., Oravecz, T., Carson, K.G.(2009) Bioorg Med Chem Lett 19: 6784-6787
- PubMed: 19836232
- DOI: https://doi.org/10.1016/j.bmcl.2009.09.081
- Primary Citation of Related Structures:
3IPX, 3IPY - PubMed Abstract:
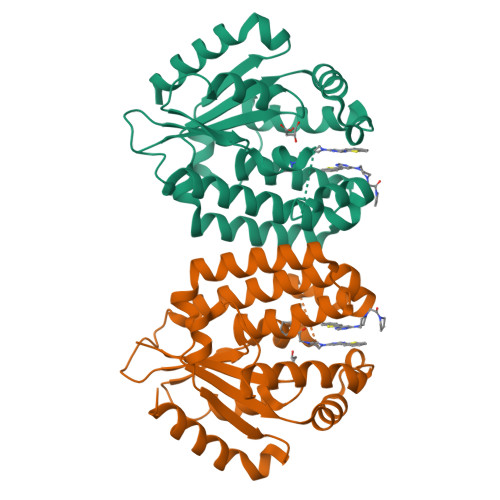
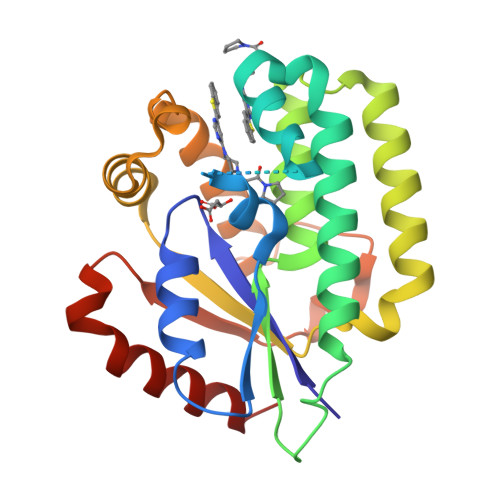
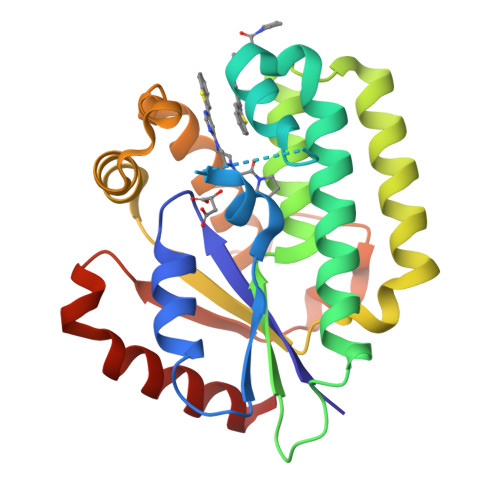
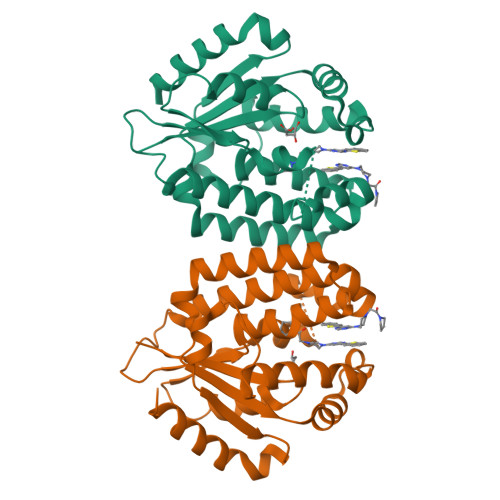
A series of deoxycytidine kinase inhibitors was simultaneously optimized for potency and PK properties. A co-crystal structure then allowed merging this series with a high throughput screening hit to afford a highly potent, selective and orally bioavailable inhibitor, compound 10. This compound showed dose dependent inhibition of deoxycytidine kinase in vivo.
Organizational Affiliation:
Lexicon Pharmaceuticals, Princeton, NJ 08540, United States. tjessop@lexpharma.com