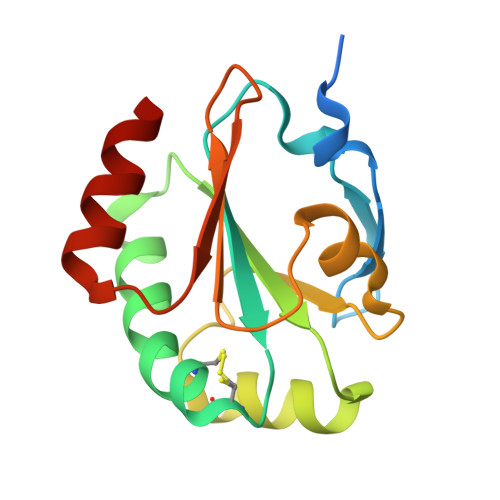
An extracellular disulfide bond forming protein (DsbF) from Mycobacterium tuberculosis: structural, biochemical, and gene expression analysis.
Chim, N., Riley, R., The, J., Im, S., Segelke, B., Lekin, T., Yu, M., Hung, L.W., Terwilliger, T., Whitelegge, J.P., Goulding, C.W.(2010) J Mol Biology 396: 1211-1226
- PubMed: 20060836
- DOI: https://doi.org/10.1016/j.jmb.2009.12.060
- Primary Citation of Related Structures:
3IOS - PubMed Abstract:
Disulfide bond forming (Dsb) proteins ensure correct folding and disulfide bond formation of secreted proteins. Previously, we showed that Mycobacterium tuberculosis DsbE (Mtb DsbE, Rv2878c) aids in vitro oxidative folding of proteins. Here, we present structural, biochemical, and gene expression analyses of another putative Mtb secreted disulfide bond isomerase protein homologous to Mtb DsbE, Mtb DsbF (Rv1677). The X-ray crystal structure of Mtb DsbF reveals a conserved thioredoxin fold although the active-site cysteines may be modeled in both oxidized and reduced forms, in contrast to the solely reduced form in Mtb DsbE. Furthermore, the shorter loop region in Mtb DsbF results in a more solvent-exposed active site. Biochemical analyses show that, similar to Mtb DsbE, Mtb DsbF can oxidatively refold reduced, unfolded hirudin and has a comparable pK(a) for the active-site solvent-exposed cysteine. However, contrary to Mtb DsbE, the Mtb DsbF redox potential is more oxidizing and its reduced state is more stable. From computational genomics analysis of the M. tuberculosis genome, we identified a potential Mtb DsbF interaction partner, Rv1676, a predicted peroxiredoxin. Complex formation is supported by protein coexpression studies and inferred by gene expression profiles, whereby Mtb DsbF and Rv1676 are upregulated under similar environments. Additionally, comparison of Mtb DsbF and Mtb DsbE gene expression data indicates anticorrelated gene expression patterns, suggesting that these two proteins and their functionally linked partners constitute analogous pathways that may function under different conditions.
Organizational Affiliation:
Department of Molecular Biology and Biochemistry, UCI, Irvine, CA 92697, USA.