Structural characterisation of ligand-binding determinants in human lung surfactant protein D: influence of Asp325
Shrive, A.K., Martin, C., Burns, I., Paterson, J.M., Martin, J.D., Townsend, J.P., Waters, P., Clark, H.W., Kishore, U., Reid, K.B.M., Greenhough, T.J.(2009) J Mol Biology 394: 776-788
- PubMed: 19799916
- DOI: https://doi.org/10.1016/j.jmb.2009.09.057
- Primary Citation of Related Structures:
3IKN, 3IKP, 3IKQ, 3IKR - PubMed Abstract:
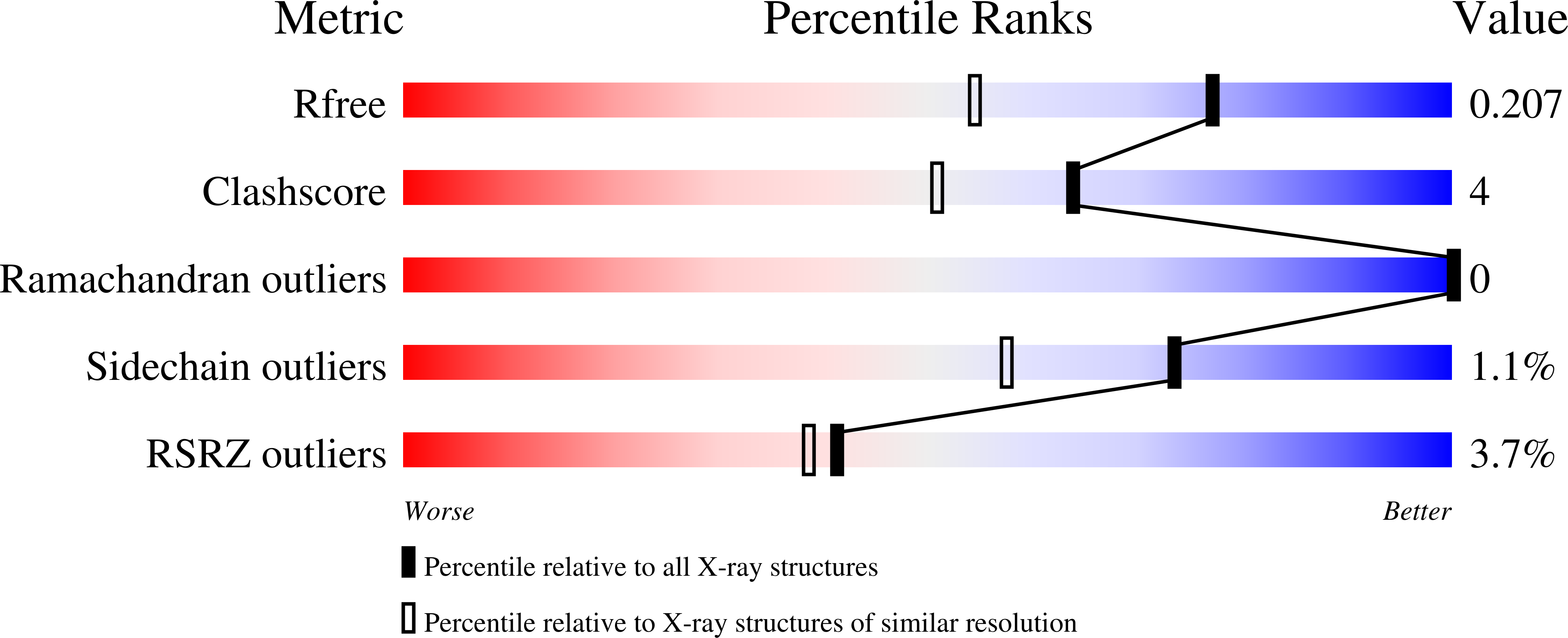
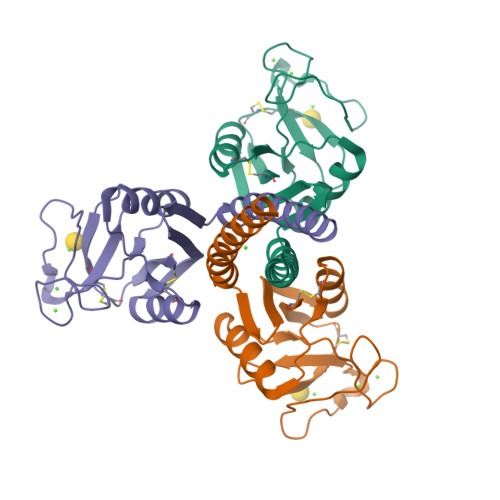
The crystal structures of a biologically and therapeutically active recombinant homotrimeric fragment of human lung surfactant protein D with a series of bound ligands have been determined. While the structures reveal various different binding modes, all utilise a similarly positioned pair of mannose-type O3' and O4' hydroxyls with no direct interaction between any non-terminal sugar and protein. The orientation, position, and interactions of the bound terminal sugar depend on the sugar itself, the presence and form of glycosidic linkage, and the environment in the crystal, which, via Asp325, places stereochemical and electronic constraints, different for the three different subunits in the homotrimer, on the ligand-binding site. As a direct consequence of this influence, the other binding-pocket flanking residue, Arg343, exhibits variable conformation and variable interactions with bound ligand and leaves open to question which orientation of terminal mannobiose, and of other terminal disaccharides, may be present in extended physiological ligands. The combined structural evidence shows that there is significant flexibility in recognition; that Asp325, in addition to Arg343, is an important determinant of ligand selectivity, recognition, and binding; and that differences in crystal contact interfaces exert, through Asp325, significant influence on preferred binding modes.
Organizational Affiliation:
Research Institute of Science and Technology in Medicine, and School of Life Sciences, Keele University, Staffordshire ST5 5BG, UK. a.k.shrive@keele.ac.uk