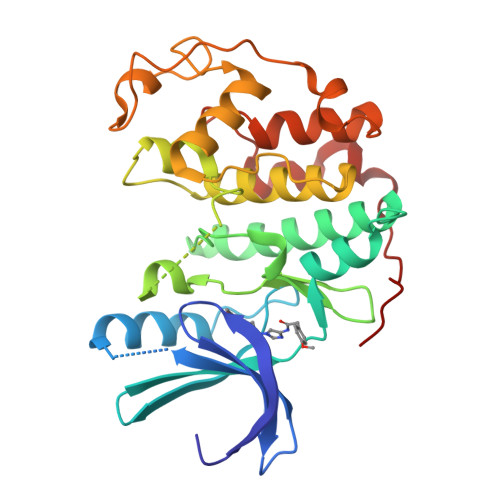
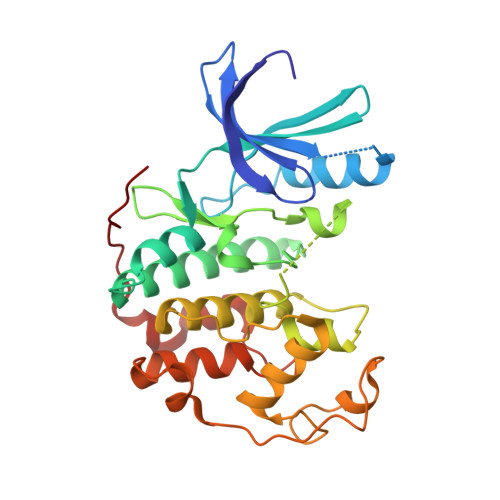
Potent and cellularly active 4-aminoimidazole inhibitors of cyclin-dependent kinase 5/p25 for the treatment of Alzheimer's disease.
Helal, C.J., Kang, Z., Lucas, J.C., Gant, T., Ahlijanian, M.K., Schachter, J.B., Richter, K.E., Cook, J.M., Menniti, F.S., Kelly, K., Mente, S., Pandit, J., Hosea, N.(2009) Bioorg Med Chem Lett 19: 5703-5707
- PubMed: 19700321
- DOI: https://doi.org/10.1016/j.bmcl.2009.08.019
- Primary Citation of Related Structures:
3IG7, 3IGG - PubMed Abstract:
Utilizing structure-based drug design, a 4-aminoimidazole heterocyclic core was synthesized as a replacement for a 2-aminothiazole due to potential metabolically mediated toxicity. The synthetic route utilized allowed for ready synthesis of 1-substituted-4-aminoimidazoles. SAR exploration resulted in the identification of a novel cis-substituted cyclobutyl group that gave improved enzyme and cellular potency against cdk5/p25 with up to 30-fold selectivity over cdk2/cyclin E.
Organizational Affiliation:
Neuroscience Medicinal Chemistry, Pfizer Global Research and Development, Eastern Point Road, Groton, CT 06340, USA. chris.j.helal@pfizer.com