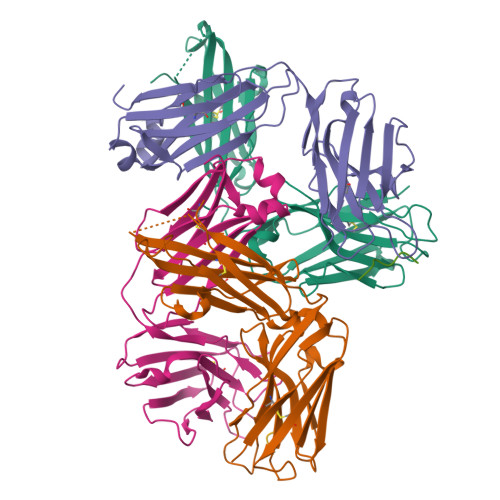
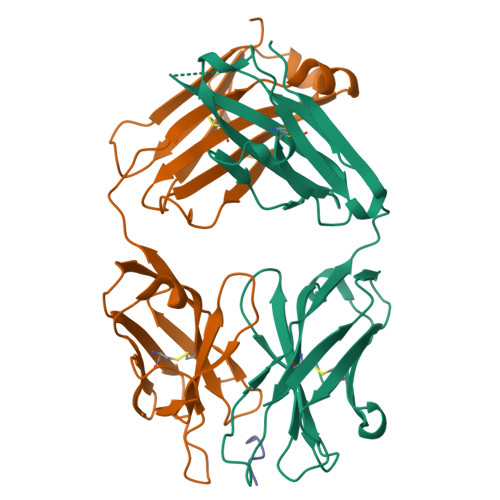
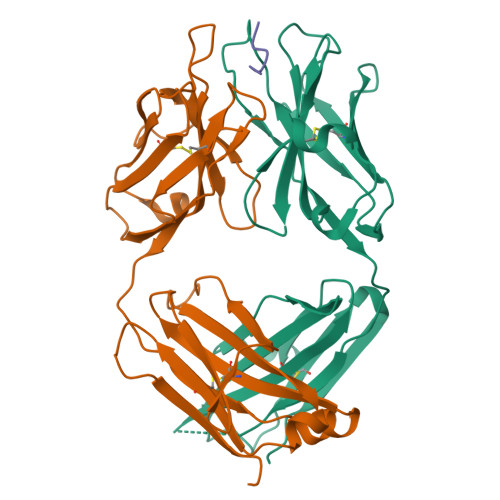
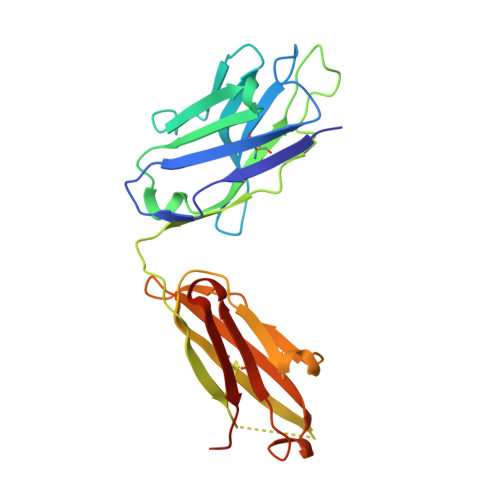
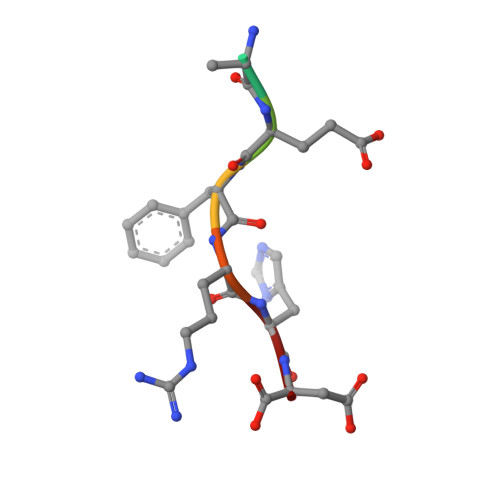
Structural correlates of antibodies associated with acute reversal of amyloid beta-related behavioral deficits in a mouse model of Alzheimer disease.
Basi, G.S., Feinberg, H., Oshidari, F., Anderson, J., Barbour, R., Baker, J., Comery, T.A., Diep, L., Gill, D., Johnson-Wood, K., Goel, A., Grantcharova, K., Lee, M., Li, J., Partridge, A., Griswold-Prenner, I., Piot, N., Walker, D., Widom, A., Pangalos, M.N., Seubert, P., Jacobsen, J.S., Schenk, D., Weis, W.I.(2010) J Biological Chem 285: 3417-3427
- PubMed: 19923222
- DOI: https://doi.org/10.1074/jbc.M109.045187
- Primary Citation of Related Structures:
3IFL, 3IFN, 3IFO, 3IFP - PubMed Abstract:
Immunotherapy targeting of amyloid beta (Abeta) peptide in transgenic mouse models of Alzheimer disease (AD) has been widely demonstrated to resolve amyloid deposition as well as associated neuronal, glial, and inflammatory pathologies. These successes have provided the basis for ongoing clinical trials of immunotherapy for treatment of AD in humans. Acute as well as chronic Abeta-targeted immunotherapy has also been demonstrated to reverse Abeta-related behavioral deficits assessing memory in AD transgenic mouse models. We observe that three antibodies targeting the same linear epitope of Abeta, Abeta(3-7), differ in their ability to reverse contextual fear deficits in Tg2576 mice in an acute testing paradigm. Reversal of contextual fear deficit by the antibodies does not correlate with in vitro recognition of Abeta in a consistent or correlative manner. To better define differences in antigen recognition at the atomic level, we determined crystal structures of Fab fragments in complex with Abeta. The conformation of the Abeta peptide recognized by all three antibodies was highly related and is also remarkably similar to that observed in independently reported Abeta:antibody crystal structures. Sequence and structural differences between the antibodies, particularly in CDR3 of the heavy chain variable region, are proposed to account for differing in vivo properties of the antibodies under study. These findings provide a structural basis for immunotherapeutic strategies targeting Abeta species postulated to underlie cognitive deficits in AD.
Organizational Affiliation:
Elan Pharmaceuticals, Incorporated, South San Francisco, California 94080, USA. guriq.basi@elan.com