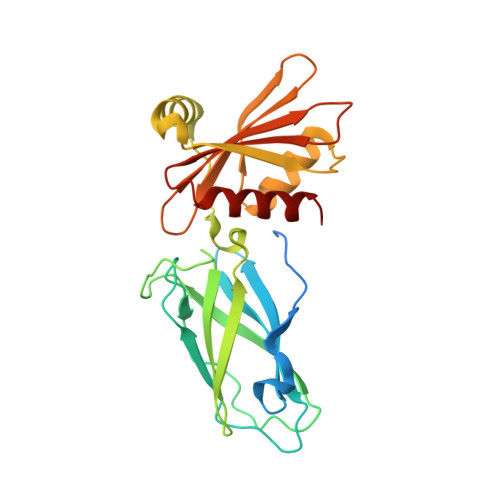
Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2.
Pechstein, A., Bacetic, J., Vahedi-Faridi, A., Gromova, K., Sundborger, A., Tomlin, N., Krainer, G., Vorontsova, O., Schafer, J.G., Owe, S.G., Cousin, M.A., Saenger, W., Shupliakov, O., Haucke, V.(2010) Proc Natl Acad Sci U S A 107: 4206-4211
- PubMed: 20160082
- DOI: https://doi.org/10.1073/pnas.0911073107
- Primary Citation of Related Structures:
3HS8, 3HS9 - PubMed Abstract:
Clathrin-mediated synaptic vesicle (SV) recycling involves the spatiotemporally controlled assembly of clathrin coat components at phosphatidylinositiol (4, 5)-bisphosphate [PI(4,5)P(2)]-enriched membrane sites within the periactive zone. Such spatiotemporal control is needed to coordinate SV cargo sorting with clathrin/AP2 recruitment and to restrain membrane fission and synaptojanin-mediated uncoating until membrane deformation and clathrin coat assembly are completed. The molecular events underlying these control mechanisms are unknown. Here we show that the endocytic SH3 domain-containing accessory protein intersectin 1 scaffolds the endocytic process by directly associating with the clathrin adaptor AP2. Acute perturbation of the intersectin 1-AP2 interaction in lamprey synapses in situ inhibits the onset of SV recycling. Structurally, complex formation can be attributed to the direct association of hydrophobic peptides within the intersectin 1 SH3A-B linker region with the "side sites" of the AP2 alpha- and beta-appendage domains. AP2 appendage association of the SH3A-B linker region inhibits binding of the inositol phosphatase synaptojanin 1 to intersectin 1. These data identify the intersectin-AP2 complex as an important regulator of clathrin-mediated SV recycling in synapses.
Organizational Affiliation:
Institute of Chemistry and Biochemistry, Freie Universität and Charité Universitätsmedizin Berlin, 14195 Berlin, Germany.