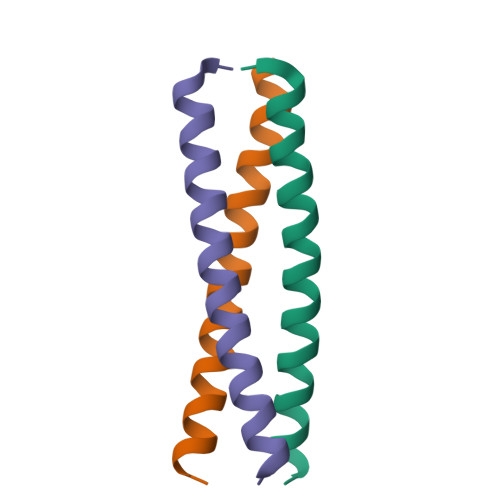
Structural and molecular basis of the assembly of the TRPP2/PKD1 complex.
Yu, Y., Ulbrich, M.H., Li, M.H., Buraei, Z., Chen, X.Z., Ong, A.C., Tong, L., Isacoff, E.Y., Yang, J.(2009) Proc Natl Acad Sci U S A 106: 11558-11563
- PubMed: 19556541
- DOI: https://doi.org/10.1073/pnas.0903684106
- Primary Citation of Related Structures:
3HRN, 3HRO - PubMed Abstract:
Mutations in PKD1 and TRPP2 account for nearly all cases of autosomal dominant polycystic kidney disease (ADPKD). These 2 proteins form a receptor/ion channel complex on the cell surface. Using a combination of biochemistry, crystallography, and a single-molecule method to determine the subunit composition of proteins in the plasma membrane of live cells, we find that this complex contains 3 TRPP2 and 1 PKD1. A newly identified coiled-coil domain in the C terminus of TRPP2 is critical for the formation of this complex. This coiled-coil domain forms a homotrimer, in both solution and crystal structure, and binds to a single coiled-coil domain in the C terminus of PKD1. Mutations that disrupt the TRPP2 coiled-coil domain trimer abolish the assembly of both the full-length TRPP2 trimer and the TRPP2/PKD1 complex and diminish the surface expression of both proteins. These results have significant implications for the assembly, regulation, and function of the TRPP2/PKD1 complex and the pathogenic mechanism of some ADPKD-producing mutations.
Organizational Affiliation:
Department of Biological Sciences, Columbia University, New York, NY 10027, USA.