Structure-function analyses of isochorismate-pyruvate lyase from Pseudomonas aeruginosa suggest differing catalytic mechanisms for the two pericyclic reactions of this bifunctional enzyme.
Luo, Q., Olucha, J., Lamb, A.L.(2009) Biochemistry 48: 5239-5245
- PubMed: 19432488
- DOI: https://doi.org/10.1021/bi900456e
- Primary Citation of Related Structures:
3HGW, 3HGX - PubMed Abstract:
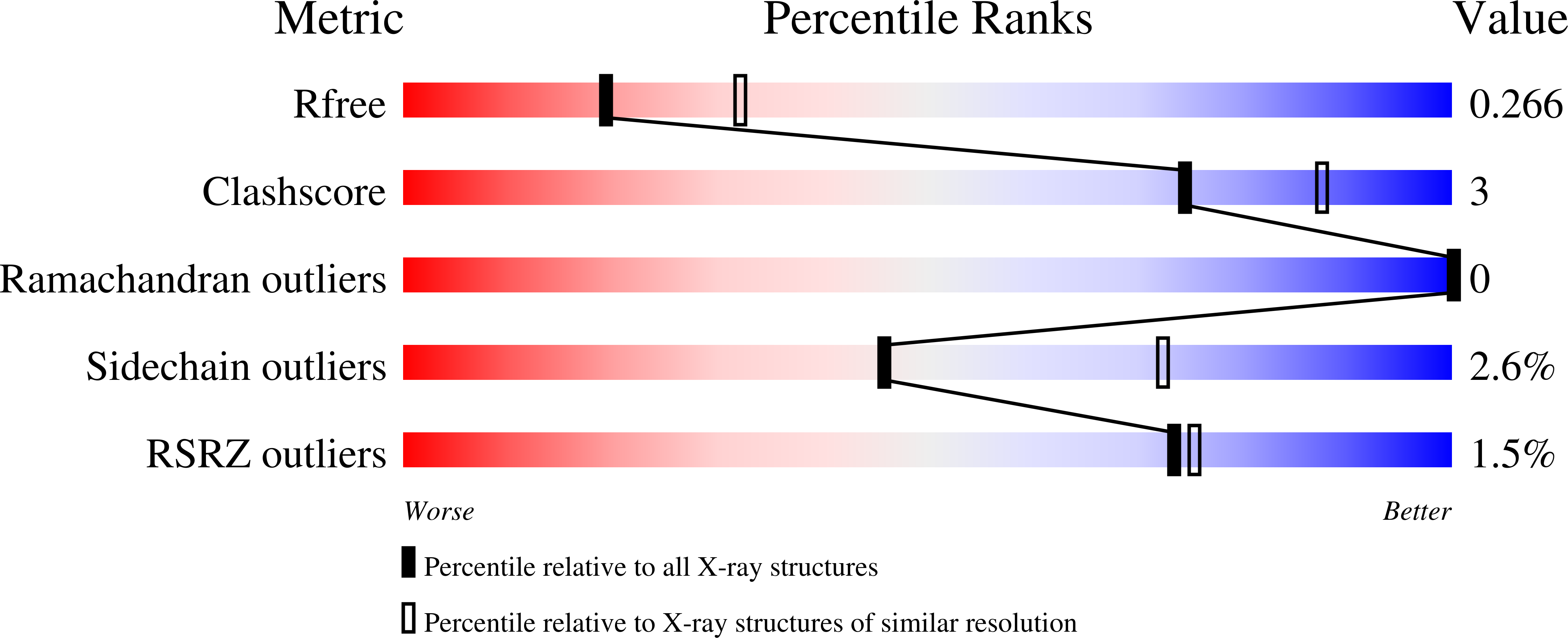
The isochorismate-pyruvate lyase from Pseudomonas aeruginosa (PchB) catalyzes two pericyclic reactions in a single active site. PchB physiologically produces salicylate and pyruvate from isochorismate for ultimate incorporation of the salicylate into the siderophore pyochelin. PchB also produces prephenate from chorismate, most likely due to structural homology to the Escherchia coli chorismate mutase. The molecular basis of catalysis among enzymatic pericyclic reactions is a matter of debate, one view holding that catalysis may be derived from electrostatic transition state stabilization and the opposing view that catalysis is derived from the generation of a reactive substrate conformation. Mutant forms of PchB were generated by site-directed mutagenesis at the site (K42) hypothesized to be key for electrostatic transition state stabilization (K42A, K42Q, K42E, and K42H). The loop containing K42 is mobile, and a mutant to slow loop dynamics was also designed (A43P). Finally, a previously characterized mutation (I87T) was also produced. Circular dichroism was used to assess the overall effect on secondary structure as a result of the mutations, and X-ray crystallographic structures are reported for K42A with salicylate and pyruvate bound and for apo-I87T. The data illustrate that the active site architecture is maintained in K42A-PchB, which indicates that differences in activity are not caused by secondary structural changes or by differences in active site loop conformation but rather by the chemical nature of this key residue. In contrast, the I87T structure demonstrates considerable mobility, suggesting that loop dynamics and conformational plasticity may be important for efficient catalysis. Finally, the mutational effects on k(cat) provide evidence that the two activities of PchB are not covariant and that a single hypothesis may not provide a sufficient explanation for catalysis.
Organizational Affiliation:
Department of Molecular Biosciences, University of Kansas, Lawrence, Kansas 66045, USA.