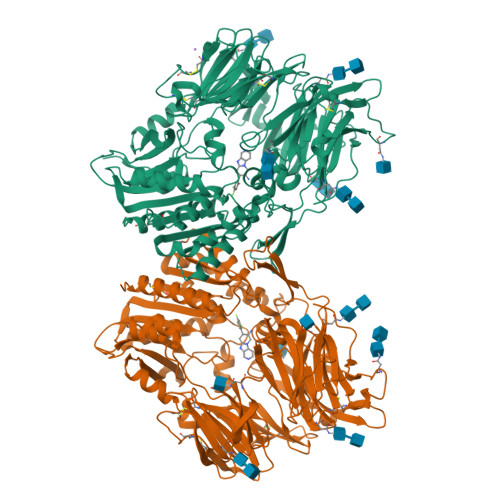
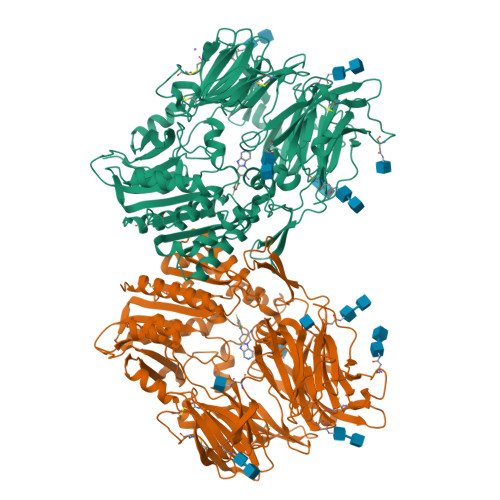
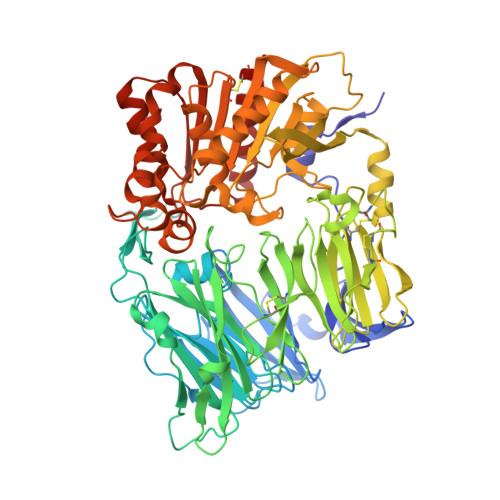
Aminopiperidine-fused imidazoles as dipeptidyl peptidase-IV inhibitors
Edmondson, S.D., Mastracchio, A., Cox, J.M., Eiermann, G.J., He, H., Lyons, K.A., Patel, R.A., Patel, S.B., Petrov, A., Scapin, G., Wu, J.K., Xu, S., Zhu, B., Thornberry, N.A., Roy, R.S., Weber, A.E.(2009) Bioorg Med Chem Lett 19: 4097-4101
- PubMed: 19539471
- DOI: https://doi.org/10.1016/j.bmcl.2009.06.011
- Primary Citation of Related Structures:
3HAB, 3HAC - PubMed Abstract:
A new series of DPP-4 inhibitors derived from piperidine-fused benzimidazoles and imidazopyridines is described. Optimization of this class of DPP-4 inhibitors led to the discovery of imidazopyridine 34. The potency, selectivity, cross-species DMPK profiles, and in vivo efficacy of 34 is reported.
Organizational Affiliation:
Department of Medicinal Chemistry, Merck & Co. Inc., PO Box 2000, Rahway, NJ 07065, USA. scott_edmondson@merck.com