Design, synthesis and selection of DNA-encoded small-molecule libraries.
Clark, M.A., Acharya, R.A., Arico-Muendel, C.C., Belyanskaya, S.L., Benjamin, D.R., Carlson, N.R., Centrella, P.A., Chiu, C.H., Creaser, S.P., Cuozzo, J.W., Davie, C.P., Ding, Y., Franklin, G.J., Franzen, K.D., Gefter, M.L., Hale, S.P., Hansen, N.J., Israel, D.I., Jiang, J., Kavarana, M.J., Kelley, M.S., Kollmann, C.S., Li, F., Lind, K., Mataruse, S., Medeiros, P.F., Messer, J.A., Myers, P., O'Keefe, H., Oliff, M.C., Rise, C.E., Satz, A.L., Skinner, S.R., Svendsen, J.L., Tang, L., van Vloten, K., Wagner, R.W., Yao, G., Zhao, B., Morgan, B.A.(2009) Nat Chem Biol 5: 647-654
- PubMed: 19648931
- DOI: https://doi.org/10.1038/nchembio.211
- Primary Citation of Related Structures:
3HA6, 3HA8 - PubMed Abstract:
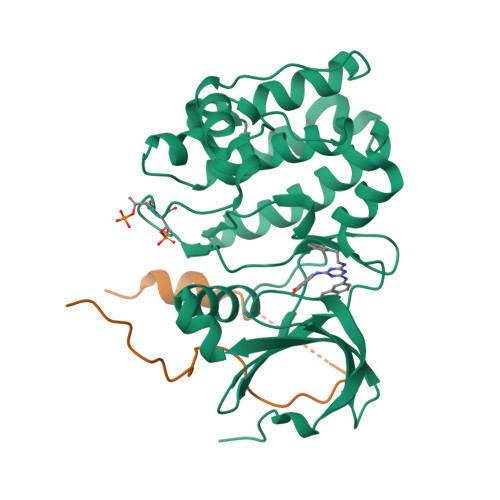
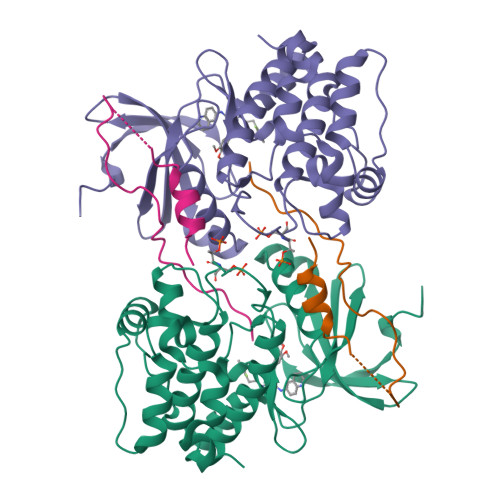
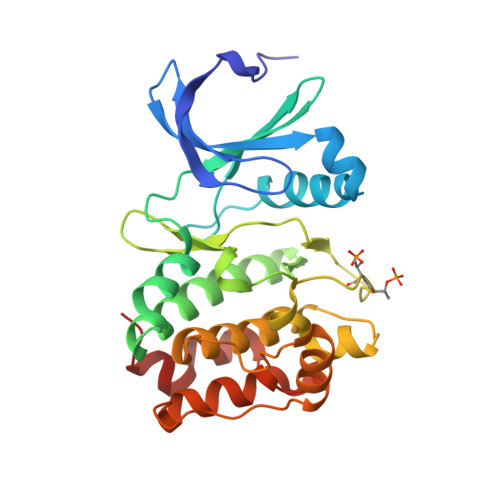
Biochemical combinatorial techniques such as phage display, RNA display and oligonucleotide aptamers have proven to be reliable methods for generation of ligands to protein targets. Adapting these techniques to small synthetic molecules has been a long-sought goal. We report the synthesis and interrogation of an 800-million-member DNA-encoded library in which small molecules are covalently attached to an encoding oligonucleotide. The library was assembled by a combination of chemical and enzymatic synthesis, and interrogated by affinity selection. We describe methods for the selection and deconvolution of the chemical display library, and the discovery of inhibitors for two enzymes: Aurora A kinase and p38 MAP kinase.
Organizational Affiliation:
Praecis Pharmaceuticals, Waltham, Massachusetts, USA