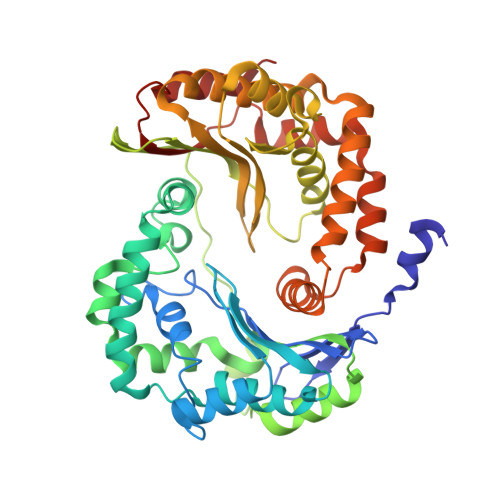
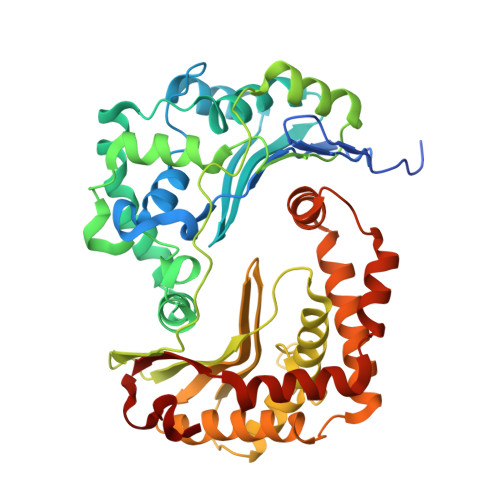
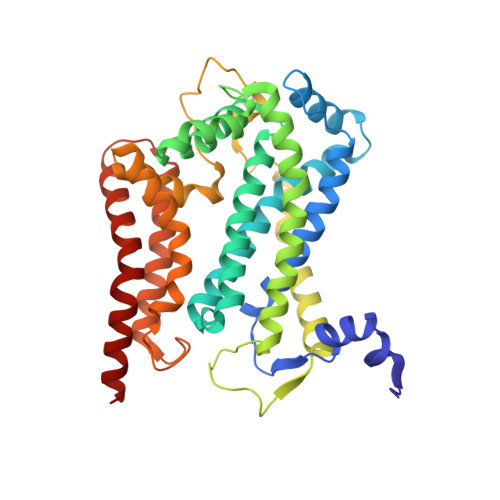
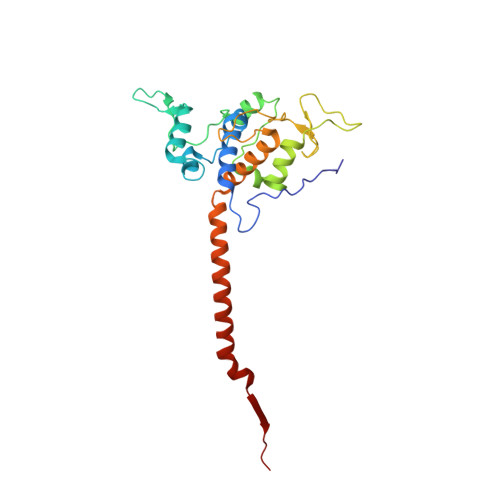
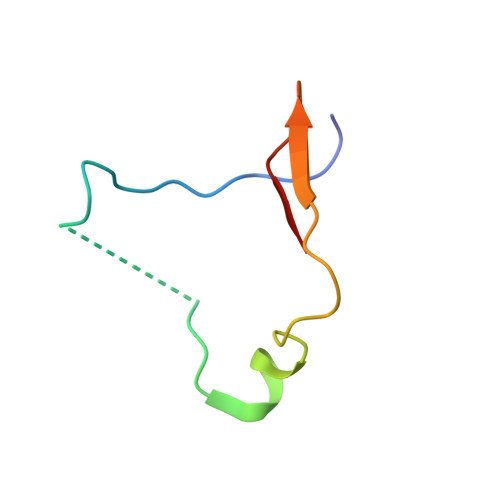
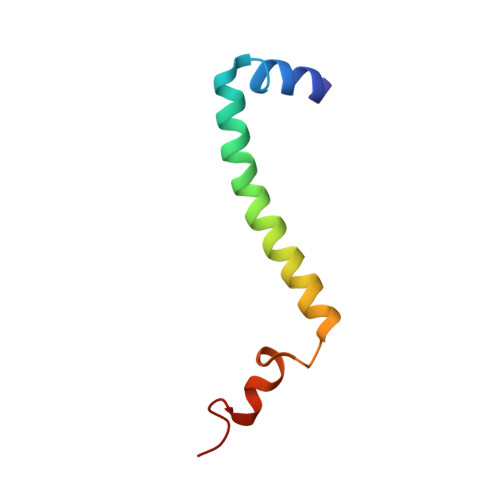
Electron Transfer by Domain Movement in Cytochrome Bc1
Zhang, Z., Huang, L.-S., Shulmeister, V.M., Chi, Y.I., Kim, K.K., Hung, L.W., Crofts, A.R., Berry, E.A., Kim, S.-H.(1998) Nature 392: 677-684
- PubMed: 9565029
- DOI: https://doi.org/10.1038/33612
- Primary Citation of Related Structures:
1BCC, 2BCC, 3BCC, 3H1H, 3H1I, 3H1J - PubMed Abstract:
The cytochrome bc1 is one of the three major respiratory enzyme complexes residing in the inner mitochondrial membrane. Cytochrome bc1 transfers electrons from ubiquinol to cytochrome c and uses the energy thus released to form an electrochemical gradient across the inner membrane. Our X-ray crystal structures of the complex from chicken, cow and rabbit in both the presence and absence of inhibitors of quinone oxidation, reveal two different locations for the extrinsic domain of one component of the enzyme, an iron-sulphur protein. One location is close enough to the supposed quinol oxidation site to allow reduction of the Fe-S protein by ubiquinol. The other site is close enough to cytochrome c1 to allow oxidation of the Fe-S protein by the cytochrome. As neither location will allow both reactions to proceed at a suitable rate, the reaction mechanism must involve movement of the extrinsic domain of the Fe-S component in order to shuttle electrons from ubiquinol to cytochrome c1. Such a mechanism has not previously been observed in redox protein complexes.
Organizational Affiliation:
E. O. Lawrence Berkeley National Laboratory, University of California, 94720, USA.