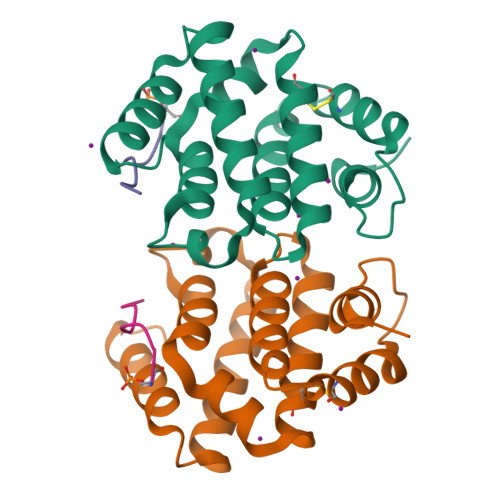
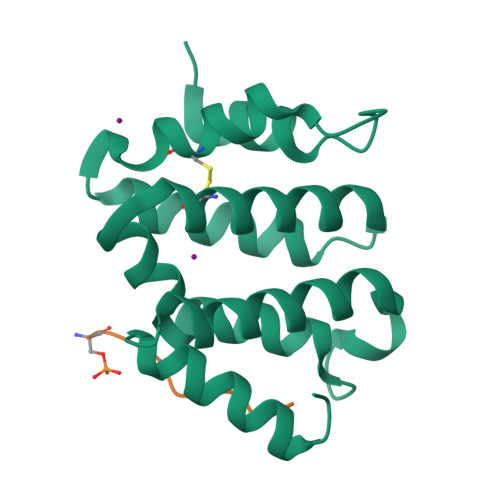
GGA autoinhibition revisited
Cramer, J.F., Gustafsen, C., Behrens, M.A., Oliveira, C.L.P., Pedersen, J.S., Madsen, P., Petersen, C.M., Thirup, S.S.(2010) Traffic 11: 259-273
- PubMed: 20015111
- DOI: https://doi.org/10.1111/j.1600-0854.2009.01017.x
- Primary Citation of Related Structures:
3G2S, 3G2T, 3G2U, 3G2V, 3G2W - PubMed Abstract:
The cytosolic adaptors GGA1-3 mediate sorting of transmembrane proteins displaying a C-terminal acidic dileucine motif (DXXLL) in their cytosolic domain. GGA1 and GGA3 contain similar but intrinsic motifs that are believed to serve as autoinhibitory sites activated by the phosphorylation of a serine positioned three residues upstream of the DXXLL motif. In the present study, we have subjected the widely acknowledged concept of GGA1 autoinhibition to a thorough structural and functional examination. We find that (i) the intrinsic motif of GGA1 is inactive, (ii) only C-terminal DXXLL motifs constitute active GGA binding sites, (iii) while aspartates and phosphorylated serines one or two positions upstream of the DXXLL motif increase GGA1 binding, phosphoserines further upstream have little or no influence and (iv) phosphorylation of GGA1 does not affect its conformation or binding to Sortilin and SorLA. Taken together, our findings seem to refute the functional significance of GGA autoinhibition in particular and of intrinsic GGA binding motifs in general.
Organizational Affiliation:
MIND Centre, Department of Molecular Biology, Aarhus University, Gustav Wieds Vej 10 C, DK-8000 Aarhus C, Denmark.