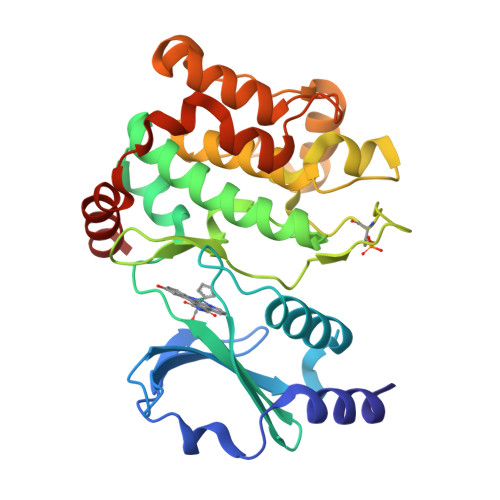
Targeting Large Kinase Active Site with Rigid, Bulky Octahedral Ruthenium Complexes
Maksimoska, J., Feng, L., Harms, K., Yi, C., Kissil, J., Marmorstein, R., Meggers, E.(2008) J Am Chem Soc 130: 15764-15765
- PubMed: 18973295
- DOI: https://doi.org/10.1021/ja805555a
- Primary Citation of Related Structures:
3FXZ, 3FY0 - PubMed Abstract:
A strategy for targeting protein kinases with large ATP-binding sites by using bulky and rigid octahedral ruthenium complexes as structural scaffolds is presented. A highly potent and selective GSK3 and Pim1 half-sandwich complex NP309 was successfully converted into a PAK1 inhibitor by making use of the large octahedral compounds Lambda-FL172 and Lambda-FL411 in which the cyclopentadienyl moiety of NP309 is replaced by a chloride and sterically demanding diimine ligands. A 1.65 A cocrystal structure of PAK1 with Lambda-FL172 reveals how the large coordination sphere of the ruthenium complex matches the size of the active site and serves as a yardstick to discriminate between otherwise closely related binding sites.
Organizational Affiliation:
The Wistar Institute and University of Pennsylvania, Department of Chemistry, Philadelphia, Pennsylvania 19104, USA.