De novo sulfur SAD phasing of the lysosomal 66.3 kDa protein from mouse
Lakomek, K., Dickmanns, A., Mueller, U., Kollmann, K., Deuschl, F., Berndt, A., Lubke, T., Ficner, R.(2009) Acta Crystallogr D Biol Crystallogr 65: 220-228
- PubMed: 19237744
- DOI: https://doi.org/10.1107/S0907444908041814
- Primary Citation of Related Structures:
3FBX - PubMed Abstract:
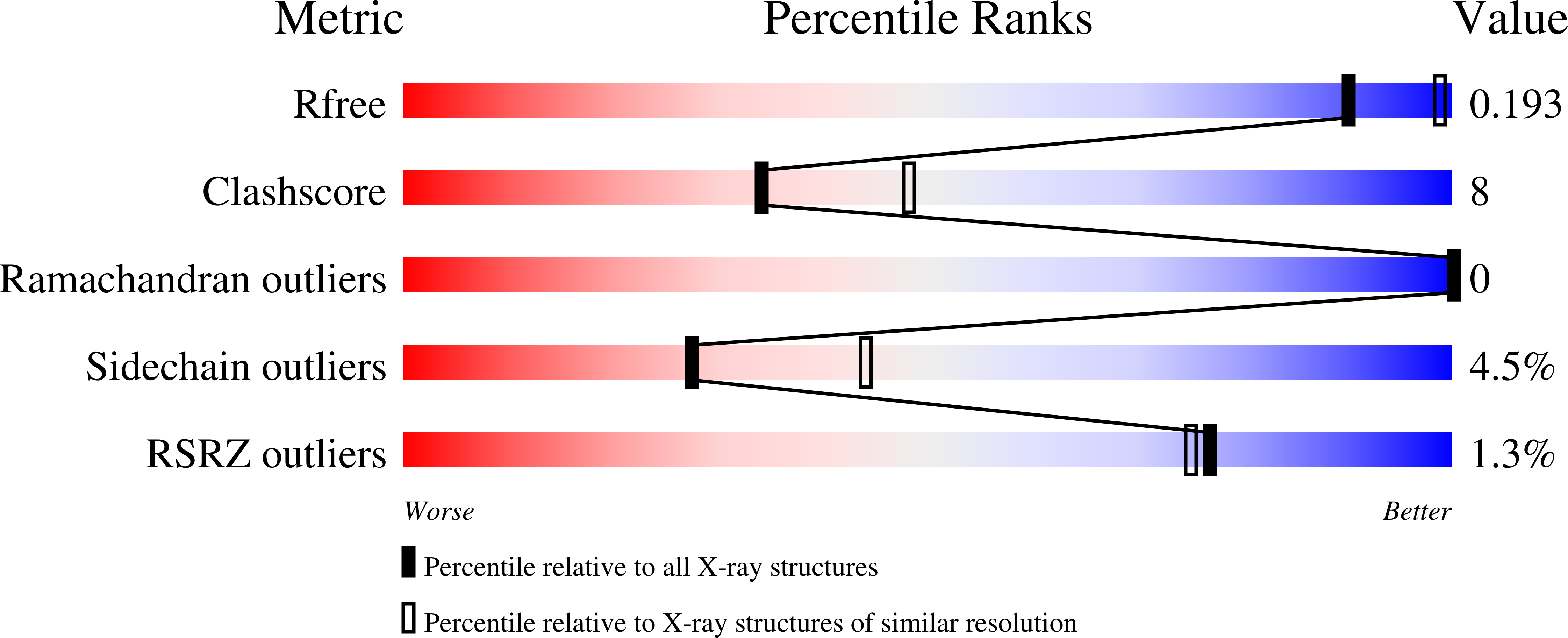
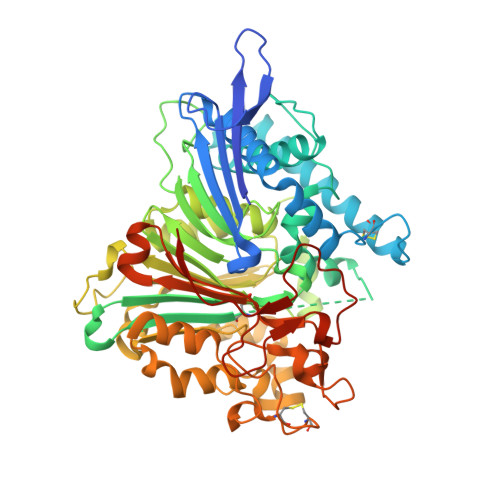
The 66.3 kDa protein from mouse is a soluble protein of the lysosomal matrix. It is synthesized as a glycosylated 75 kDa preproprotein which is further processed into 28 and 40 kDa fragments. Despite bioinformatics approaches and molecular characterization of the 66.3 kDa protein, the mode of its maturation as well as its physiological function remained unknown. Therefore, it was decided to tackle this question by means of X-ray crystallography. After expression in a human fibrosarcoma cell line, the C-terminally His-tagged single-chain 66.3 kDa variant and the double-chain form consisting of a 28 kDa fragment and a 40 kDa fragment were purified to homogeneity but could not be separated during the purification procedure. This mixture was therefore used for crystallization. Single crystals were obtained and the structure of the 66.3 kDa protein was solved by means of sulfur SAD phasing using data collected at a wavelength of 1.9 A on the BESSY beamline BL14.2 of Freie Universität Berlin. Based on the anomalous signal, a 22-atom substructure comprising 21 intrinsic S atoms and one Xe atom with very low occupancy was found and refined at a resolution of 2.4 A using the programs SHELXC/D and SHARP. Density modification using SOLOMON and DM resulted in a high-quality electron-density map, enabling automatic model building with ARP/wARP. The initial model contained 85% of the amino-acid residues expected to be present in the asymmetric unit of the crystal. Subsequently, the model was completed and refined to an R(free) factor of 19.8%. The contribution of the single Xe atom to the anomalous signal was analyzed in comparison to that of the S atoms and was found to be negligible. This work should encourage the use of the weak anomalous scattering of intrinsic S atoms in SAD phasing, especially for proteins, which require both expensive and time-consuming expression and purification procedures, preventing extensive screening of heavy-atom crystal soaks.
Organizational Affiliation:
Department of Molecular Structural Biology, Institute of Microbiology and Genetics, Georg-August University Göttingen, Göttingen, Germany.