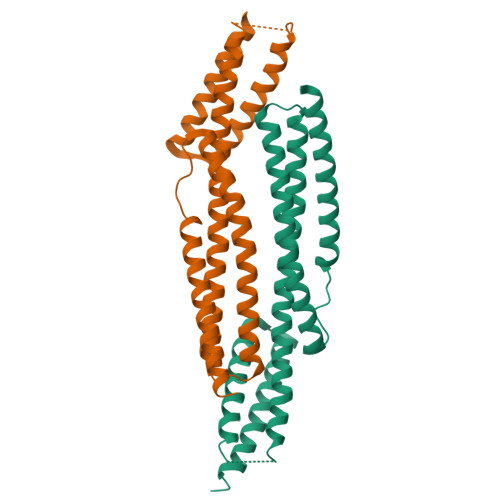
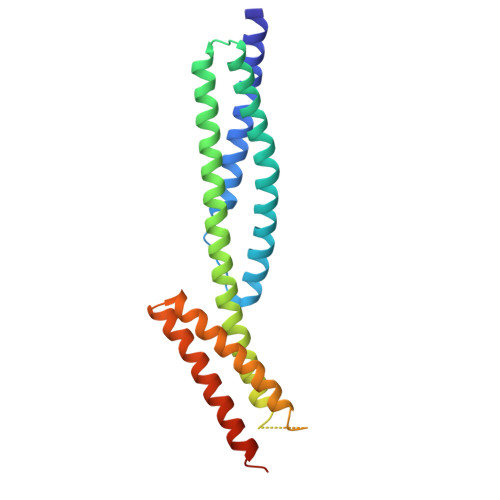
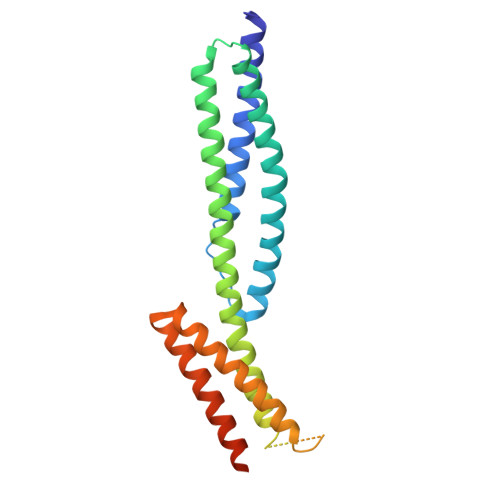
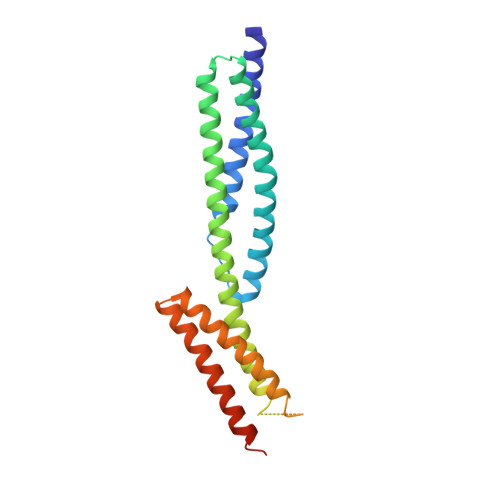
Structures of the spectrin-ankyrin interaction binding domains.
Ipsaro, J.J., Huang, L., Mondragon, A.(2009) Blood 113: 5385-5393
- PubMed: 19141864
- DOI: https://doi.org/10.1182/blood-2008-10-184358
- Primary Citation of Related Structures:
3F57, 3F59 - PubMed Abstract:
As key components of the erythrocyte membrane skeleton, spectrin and ankyrin specifically interact to tether the spectrin cytoskeleton to the cell membrane. The structure of the spectrin binding domain of ankyrin and the ankyrin binding domain of spectrin have been solved to elucidate the structural basis for ankyrin-spectrin recognition. The structure of repeats 14 and 15 of spectrin shows that these repeats are similar to all other spectrin repeats. One feature that could account for the preference of ankyrin for these repeats is the presence of a conserved, negatively charged patch on one side of repeat 14. The structure of the ankyrin ZU5 domain shows a novel structure containing a beta core. The structure reveals that the canonical ZU5 consensus sequence is likely to be missing an important region that codes for a beta strand that forms part of the core of the domain. In addition, a positively charged region is suggestive of a binding surface for the negatively charged spectrin repeat 14. Previously reported mutants of ankyrin that map to this region lie mostly on the surface of the protein, although at least one is likely to be part of the core.
Organizational Affiliation:
Department of Biochemistry, Molecular Biology, and Cell Biology, Northwestern University, Evanston, IL 60208, USA.