Carbonic anhydrase activators: kinetic and X-ray crystallographic study for the interaction of D- and L-tryptophan with the mammalian isoforms I-XIV
Temperini, C., Innocenti, A., Scozzafava, A., Supuran, C.T.(2008) Bioorg Med Chem 16: 8373-8378
- PubMed: 18774300
- DOI: https://doi.org/10.1016/j.bmc.2008.08.043
- Primary Citation of Related Structures:
3EFI - PubMed Abstract:
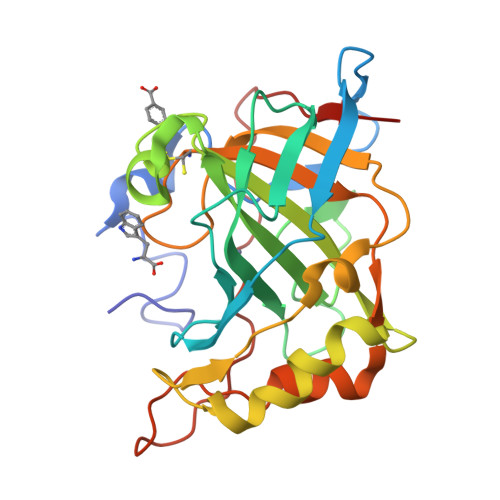
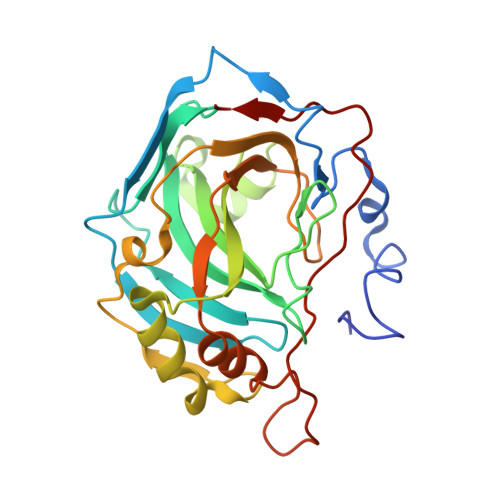
An activation study of mammalian carbonic anhydrase (CA, EC 4.2.1.1) isoforms I-XIV with D- and L-tryptophan has been performed both by means of kinetic and X-ray crystallographic techniques. These compounds show a time dependent activity against isozyme CA II, with activation constants of 1.13 microM for L-Trp and 0.37 microM for D-Trp, respectively, after 24 h of incubation between enzyme and activator. The high resolution X-ray crystal structure of the hCA II-D-Trp adduct revealed the activator to bind in a totally unprecedented way to the enzyme active site as compared to histamine, L-/D-Phe, L-/D-His or L-adrenaline. D-Trp is anchored at the edge of the CA II active site entrance, strongly interacting with amino acid residues Asp130, Phe131 and Gly132 as well as with a loop of a second symmetry related protein molecule from the asymmetric unit, by means of hydrogen bonds and several weak van der Waals interactions involving Glu234, Gly235, Glu236 and Glu238. Thus, a second activator binding site (B) within the CA II cavity has been detected, where only D-Trp was shown so far to bind, in addition to the activator binding site A, in which histamine, L-/D-Phe, and L-/D-His are bound. These findings explain the strong affinity of D-Trp for CA II and may be useful for designing novel classes of CA activators by using this compound as lead molecule.
Organizational Affiliation:
Università degli Studi di Firenze, Laboratorio di Chimica Bioinorganica, Room 188, Via della Lastruccia 3, I-50019 Sesto Fiorentino (Firenze), Italy.