Tryptophan Cu(I)-pi interaction fine-tunes the metal binding properties of the bacterial metallochaperone CusF
Loftin, I.R., Blackburn, N.J., McEvoy, M.M.(2009) J Biol Inorg Chem 14: 905-912
- PubMed: 19381697
- DOI: https://doi.org/10.1007/s00775-009-0503-y
- Primary Citation of Related Structures:
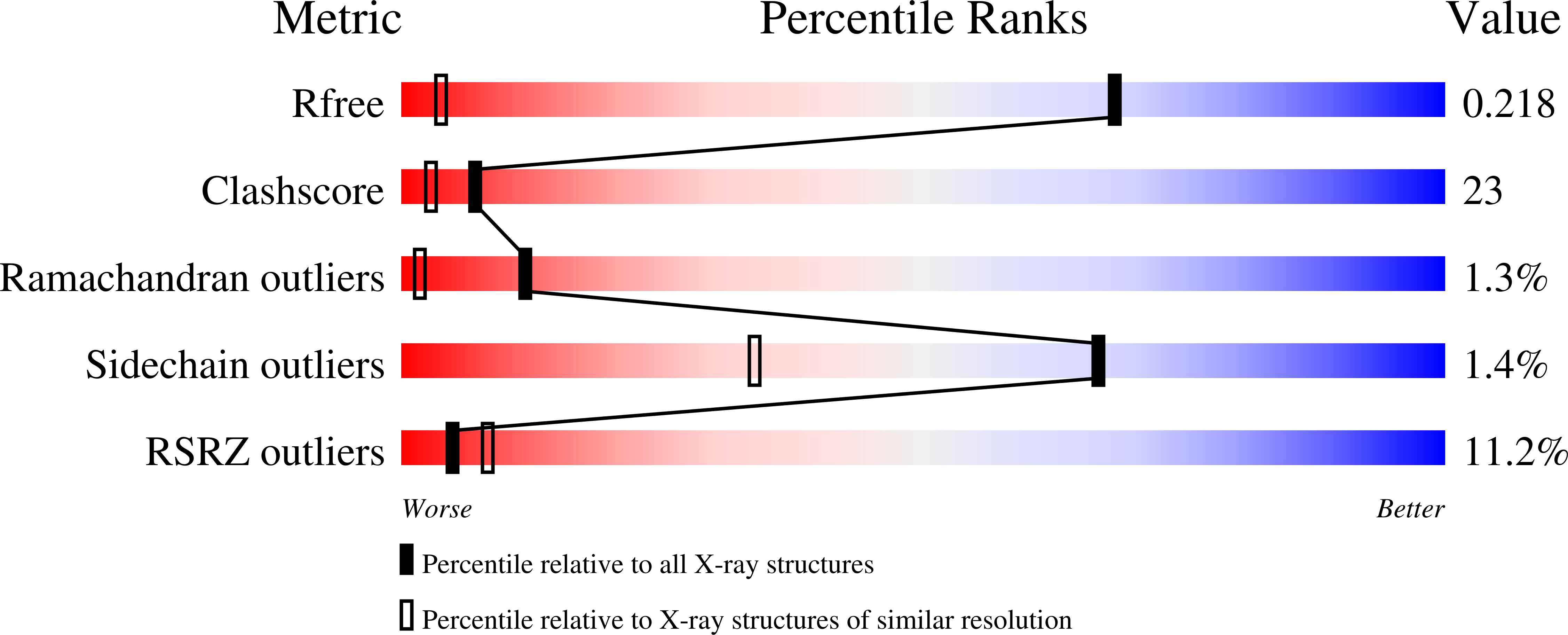
3E6Z - PubMed Abstract:
The periplasmic metallochaperone CusF coordinates Cu(I) and Ag(I) through a unique site consisting of a Met(2)His motif as well as a Cu(I)-pi interaction between a nearby tryptophan, W44, and the metal ion. Through mutational analyses we investigate here the role that W44 in CusF plays in metal coordination. Nuclear magnetic resonance spectra show that the specificity of CusF for Cu(I) and Ag(I) is not altered by mutation of W44. X-ray absorption spectroscopy studies reveal that W44 protects the bound Cu(I) from oxidation as well as from adventitious ligands. Competition assays demonstrate that W44 does not significantly contribute to the affinity of CusF for metal, but that substitution of W44 by methionine, which forms a fourth Cu(I) ligand, substantially increases the affinity. These studies indicate that W44 is important in maintaining a moderate-affinity and solvent-shielded three-coordinate environment for Cu(I), which has implications for the function of CusF as a metallochaperone.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, University of Arizona, Tucson, AZ 85721, USA.