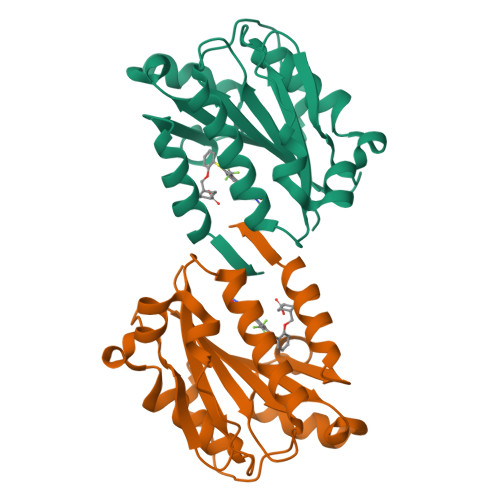
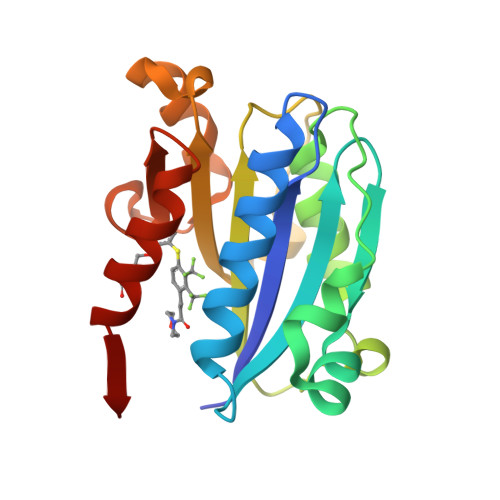
Structure-activity relationship of ortho- and meta-phenol based LFA-1 ICAM inhibitors
Lin, E.Y., Guckian, K.M., Silvian, L., Chin, D., Boriack-Sjodin, P.A., van Vlijmen, H., Friedman, J.E., Scott, D.M.(2008) Bioorg Med Chem Lett 18: 5245-5248
- PubMed: 18783948
- DOI: https://doi.org/10.1016/j.bmcl.2008.08.062
- Primary Citation of Related Structures:
3BQN, 3E2M - PubMed Abstract:
LFA-1 ICAM inhibitors based on ortho- and meta-phenol templates were designed and synthesized by Mitsunobu chemistry. The selection of targets was guided by X-ray co-crystal data, and led to compounds which showed an up to 30-fold increase in potency over reference compound 1 in the LFA-1/ICAM1-Ig assay. The most active compound exploited a new hydrogen bond to the I-domain and exhibited subnanomolar potency.
Organizational Affiliation:
Research and Development, Biogen Idec Inc., 14 Cambridge Center, Cambridge, MA 02142, USA. ted.lin@biogenidec.com