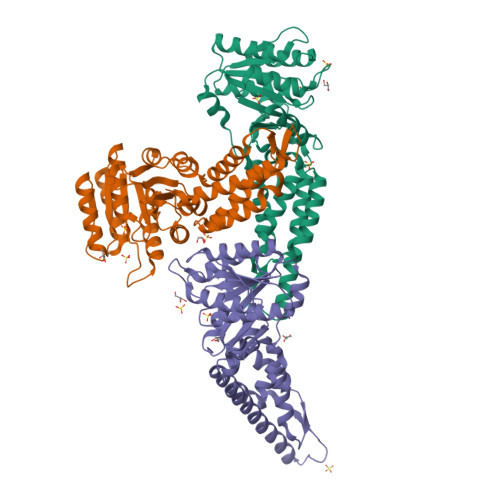
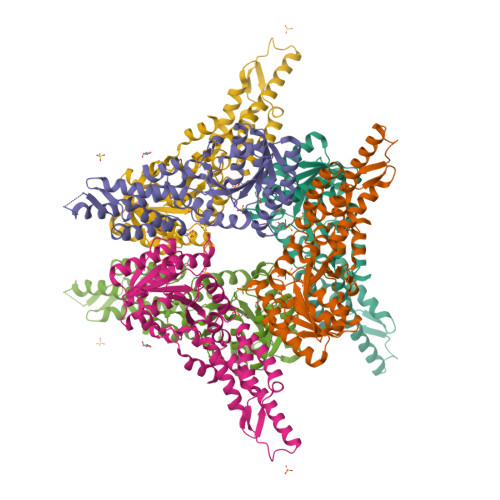
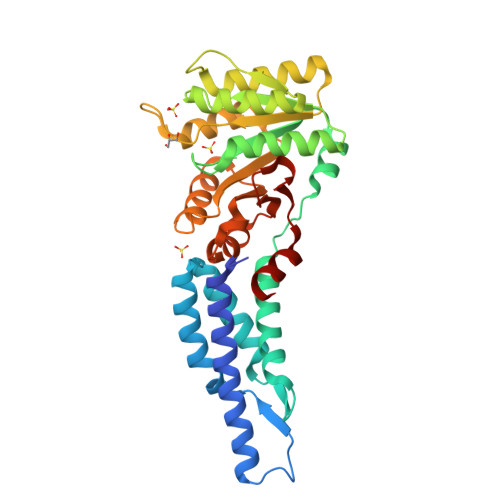
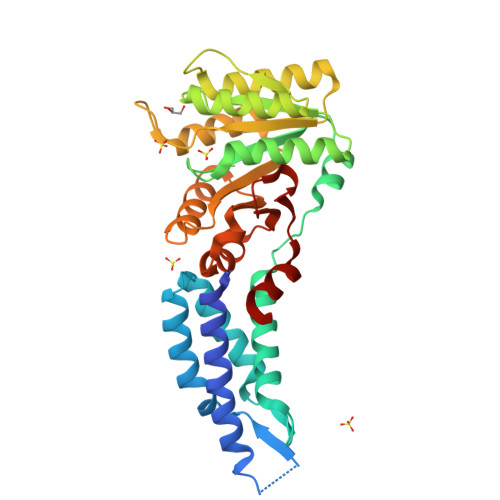
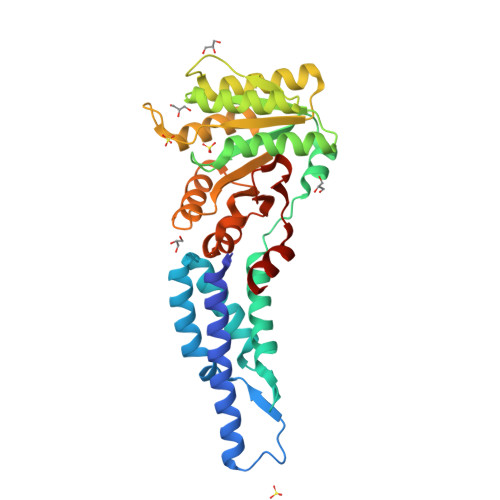
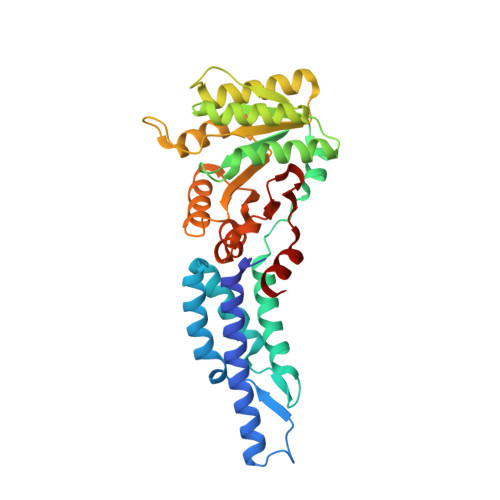
Structures of the Signal Recognition Particle Receptor from the Archaeon Pyrococcus furiosus: Implications for the Targeting Step at the Membrane.
Egea, P.F., Tsuruta, H., de Leon, G.P., Napetschnig, J., Walter, P., Stroud, R.M.(2008) PLoS One 3: e3619-e3619
- PubMed: 18978942
- DOI: https://doi.org/10.1371/journal.pone.0003619
- Primary Citation of Related Structures:
3DM9, 3DMD, 3E70 - PubMed Abstract:
In all organisms, a ribonucleoprotein called the signal recognition particle (SRP) and its receptor (SR) target nascent proteins from the ribosome to the translocon for secretion or membrane insertion. We present the first X-ray structures of an archeal FtsY, the receptor from the hyper-thermophile Pyrococcus furiosus (Pfu), in its free and GDP*magnesium-bound forms. The highly charged N-terminal domain of Pfu-FtsY is distinguished by a long N-terminal helix. The basic charges on the surface of this helix are likely to regulate interactions at the membrane. A peripheral GDP bound near a regulatory motif could indicate a site of interaction between the receptor and ribosomal or SRP RNAs. Small angle X-ray scattering and analytical ultracentrifugation indicate that the crystal structure of Pfu-FtsY correlates well with the average conformation in solution. Based on previous structures of two sub-complexes, we propose a model of the core of archeal and eukaryotic SRP*SR targeting complexes.
Organizational Affiliation:
Department of Biochemistry and Biophysics, University of California San Francisco, San Francisco, California, USA. pascal@msg.ucsf.edu