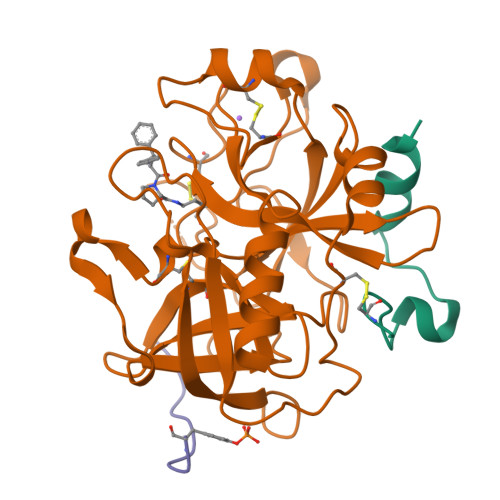
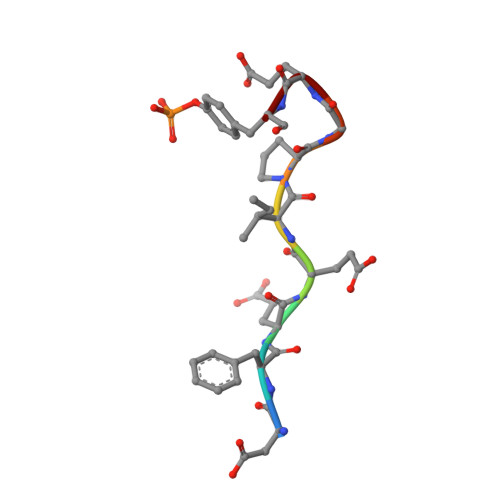
Compounds binding to the S2-S3 pockets of thrombin.
Nilsson, M., Hamalainen, M., Ivarsson, M., Gottfries, J., Xue, Y., Hansson, S., Isaksson, R., Fex, T.(2009) J Med Chem 52: 2708-2715
- PubMed: 19371038
- DOI: https://doi.org/10.1021/jm8011849
- Primary Citation of Related Structures:
3DA9 - PubMed Abstract:
A set of compounds designed to bind to the S2-S3 pockets of thrombin was prepared. These compounds included examples with no interactions in the S1 pocket. Proline, a common P2 in many thrombin inhibitors, was combined with known P3 residues and P1 substituents of varying size and lipophilicity. Binding constants were determined using surface plasmon resonance (SPR) biosensor technology and were found to be in good agreement with results from an enzyme assay. A dramatic increase in affinity (100-1000 times) was seen for compounds incorporating an amino group capable of forming a hydrogen bond with gly216 in the protein backbone. The ligand efficiency was increased by including substituents that form stronger hydrophobic interactions with the P1 pocket. The binding mode was confirmed by X-ray analysis, which revealed the anticipated binding motif that included hydrogen bonds as well as a tightly bound water molecule. A QSAR model indicated that hydrogen bonding and lipophilicity were important for the prediction of binding constants. The results described here may have implications for how directed compound libraries for shallow protein pockets, like S2 and S3 in serine proteases, can be designed.
Organizational Affiliation:
School of Pure and Applied Natural Sciences, University of Kalmar, S-391 82 Kalmar, Sweden. Mikael.Nilsson@hik.se