Structure-based inhibitor discovery of Helicobacter pylori dehydroquinate synthase.
Liu, J.S., Cheng, W.C., Wang, H.J., Chen, Y.C., Wang, W.C.(2008) Biochem Biophys Res Commun 373: 1-7
- PubMed: 18503755
- DOI: https://doi.org/10.1016/j.bbrc.2008.05.070
- Primary Citation of Related Structures:
3CLH - PubMed Abstract:
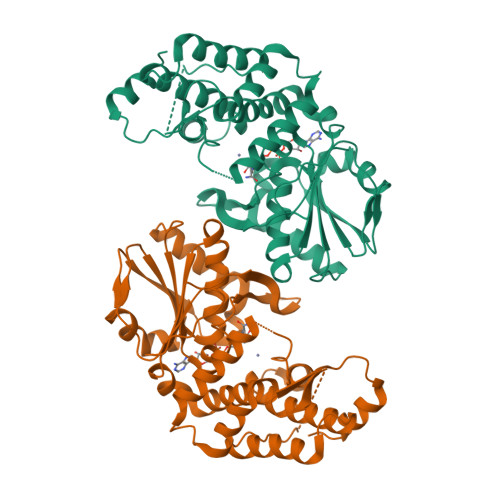
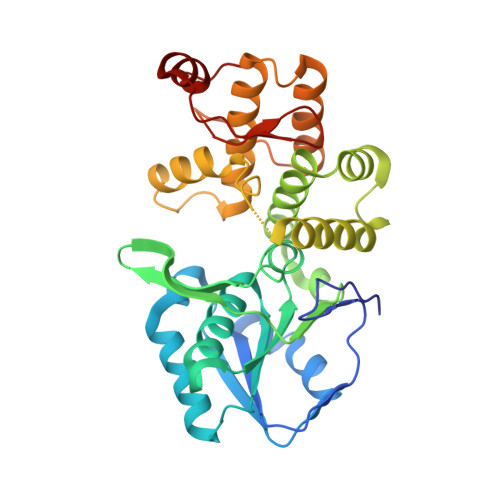
Dehydroquinate synthase (DHQS) is a nicotinamide adenine dinucleotide (NAD)-dependent enzyme that converts 3-deoxy-D-arabino-heptulosonate 7-phosphate (DAHP) into 3-dehydroquinate (DHQ). Since it catalyzes the second key step in the shikimate pathway, which is crucial for the aromatic amino acid metabolism in bacteria, fungi, and plants, but not in mammals, DHQS is a potential target for new antimicrobial agents, anti-parasitic agents and herbicides. The crystal structure of Helicobacter pylori DHQS (HpDHQS) complexed with NAD has been determined at 2.4-A resolution and was found to possess an N-terminal Rossmann-fold domain and a C-terminal alpha-helical domain. Structural comparison reveals that the binary complex adopts an open-state conformation and shares conserved residues in the binding pocket. Virtual docking of compounds into the active site of the HpDHQS structure using the GOLD docking program led to the identification of several inhibitors. The most active compound had an IC(50) value of 61 microM, which may serve as a lead for potent inhibitors.
Organizational Affiliation:
Institute of Molecular and Cellular Biology and Department of Life Sciences, National Tsing Hua University, 101, Section 2, Kuang-Fu Road, Hsinchu 300, Taiwan.