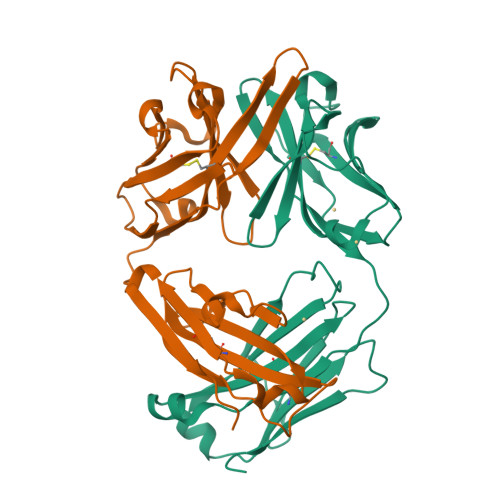
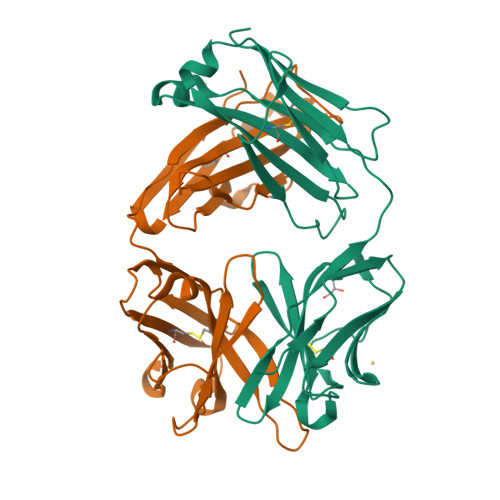
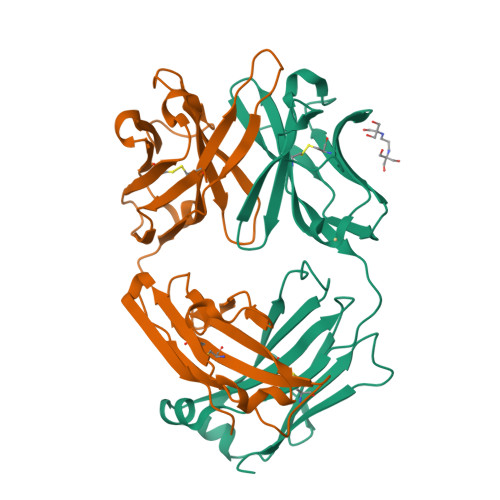
Conformational isomerism can limit antibody catalysis.
Debler, E.W., Muller, R., Hilvert, D., Wilson, I.A.(2008) J Biological Chem 283: 16554-16560
- PubMed: 18417480
- DOI: https://doi.org/10.1074/jbc.M710256200
- Primary Citation of Related Structures:
3CFJ, 3CFK - PubMed Abstract:
Ligand binding to enzymes and antibodies is often accompanied by protein conformational changes. Although such structural adjustments may be conducive to enzyme catalysis, much less is known about their effect on reactions promoted by engineered catalytic antibodies. Crystallographic and pre-steady state kinetic analyses of antibody 34E4, which efficiently promotes the conversion of benzisoxazoles to salicylonitriles, show that the resting catalyst adopts two interconverting active-site conformations, only one of which is competent to bind substrate. In the predominant isomer, the indole side chain of Trp(L91) occupies the binding site and blocks ligand access. Slow conformational isomerization of this residue, on the same time scale as catalytic turnover, creates a deep and narrow binding site that can accommodate substrate and promote proton transfer using Glu(H50) as a carboxylate base. Although 34E4 is among the best catalysts for the deprotonation of benzisoxazoles, its efficiency appears to be significantly limited by this conformational plasticity of its active site. Future efforts to improve this antibody might profitably focus on stabilizing the active conformation of the catalyst. Analogous strategies may also be relevant to other engineered proteins that are limited by an unfavorable conformational pre-equilibrium.
Organizational Affiliation:
Department of Molecular Biology and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, La Jolla, California 92037, USA.