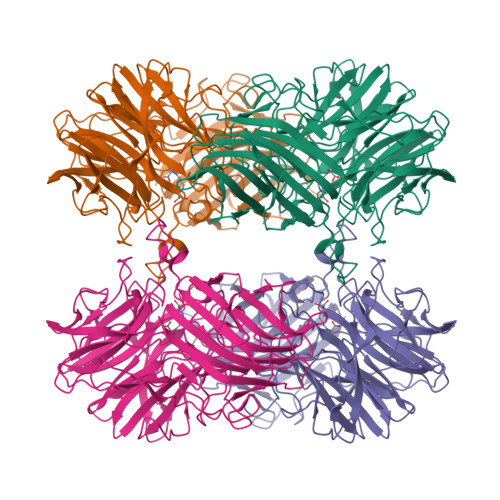
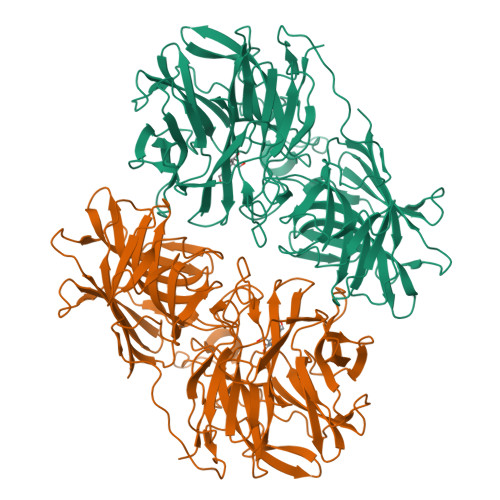
Structure of the two-subsite beta-d-xylosidase from Selenomonas ruminantium in complex with 1,3-bis[tris(hydroxymethyl)methylamino]propane.
Brunzelle, J.S., Jordan, D.B., McCaslin, D.R., Olczak, A., Wawrzak, Z.(2008) Arch Biochem Biophys 474: 157-166
- PubMed: 18374656
- DOI: https://doi.org/10.1016/j.abb.2008.03.007
- Primary Citation of Related Structures:
3C2U - PubMed Abstract:
The three-dimensional structure of the catalytically efficient beta-xylosidase from Selenomonas ruminantium in complex with competitive inhibitor 1,3-bis[tris(hydroxymethyl)methylamino]propane (BTP) was determined by using X-ray crystallography (1.3A resolution). Most H bonds between inhibitor and protein occur within subsite -1, including one between the carboxyl group of E186 and an N group of BTP. The other N of BTP occupies subsite +1 near K99. E186 (pK(a) 7.2) serves as catalytic acid. The pH (6-10) profile for 1/K(i)((BTP)) is bell-shaped with pK(a)'s 6.8 and 7.8 on the acidic limb assigned to E186 and inhibitor groups and 9.9 on the basic limb assigned to inhibitor. Mutation K99A eliminates pK(a) 7.8, strongly suggesting that the BTP monocation binds to the dianionic enzyme D14(-)E186(-). A sedimentation equilibrium experiment estimates a K(d) ([dimer](2)/[tetramer]) of 7 x 10(-9)M. Similar k(cat) and k(cat)/K(m) values were determined when the tetramer/dimer ratio changes from 0.0028 to 26 suggesting that dimers and tetramers are equally active forms.
Organizational Affiliation:
Northwestern University Center for Synchrotron Research, Life Sciences Collaborative Access Team, Department of Molecular Pharmacology and Biological Chemistry, 9700 South Cass Avenue, Argonne, IL 60439, USA.