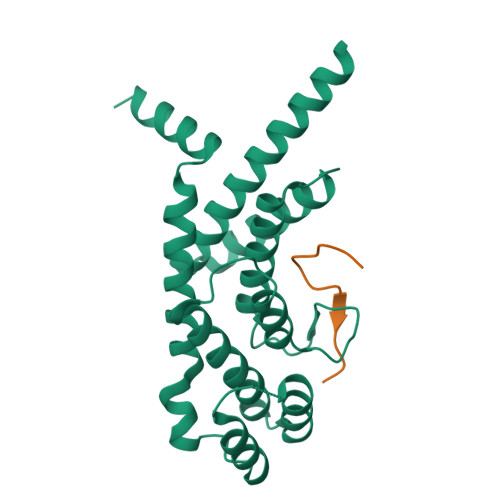
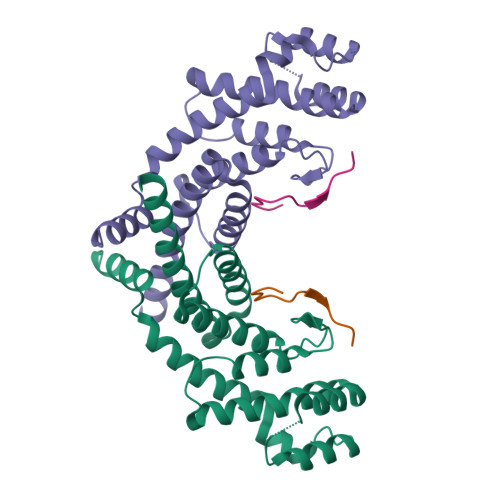
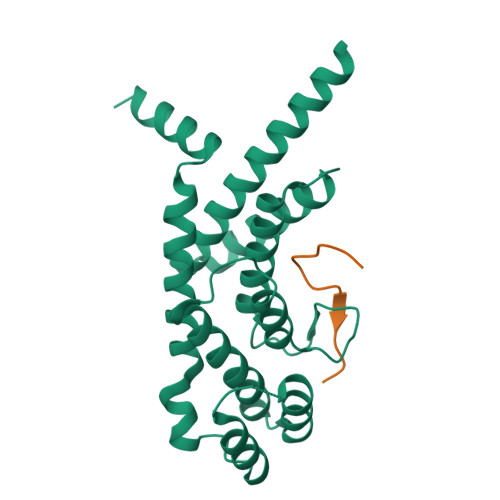
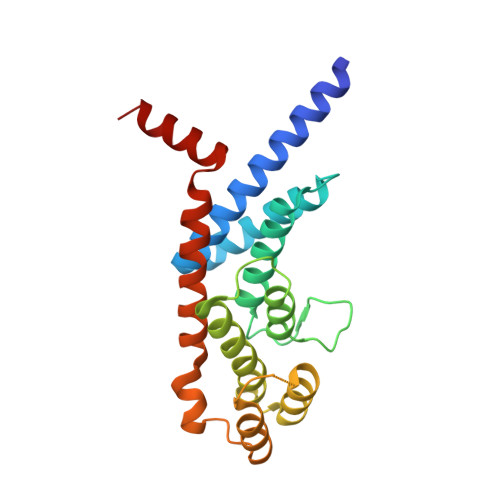
A shared docking motif in TRF1 and TRF2 used for differential recruitment of telomeric proteins.
Chen, Y., Yang, Y., van Overbeek, M., Donigian, J.R., Baciu, P., de Lange, T., Lei, M.(2008) Science 319: 1092-1096
- PubMed: 18202258
- DOI: https://doi.org/10.1126/science.1151804
- Primary Citation of Related Structures:
3BQO, 3BU8, 3BUA - PubMed Abstract:
Mammalian telomeres are protected by a six-protein complex: shelterin. Shelterin contains two closely related proteins (TRF1 and TRF2), which recruit various proteins to telomeres. We dissect the interactions of TRF1 and TRF2 with their shared binding partner (TIN2) and other shelterin accessory factors. TRF1 recognizes TIN2 using a conserved molecular surface in its TRF homology (TRFH) domain. However, this same surface does not act as a TIN2 binding site in TRF2, and TIN2 binding to TRF2 is mediated by a region outside the TRFH domain. Instead, the TRFH docking site of TRF2 binds a shelterin accessory factor (Apollo), which does not interact with the TRFH domain of TRF1. Conversely, the TRFH domain of TRF1, but not of TRF2, interacts with another shelterin-associated factor: PinX1.
Organizational Affiliation:
Department of Biological Chemistry, University of Michigan Medical School, 1150 West Medical Center Drive, Ann Arbor, MI 48109, USA.