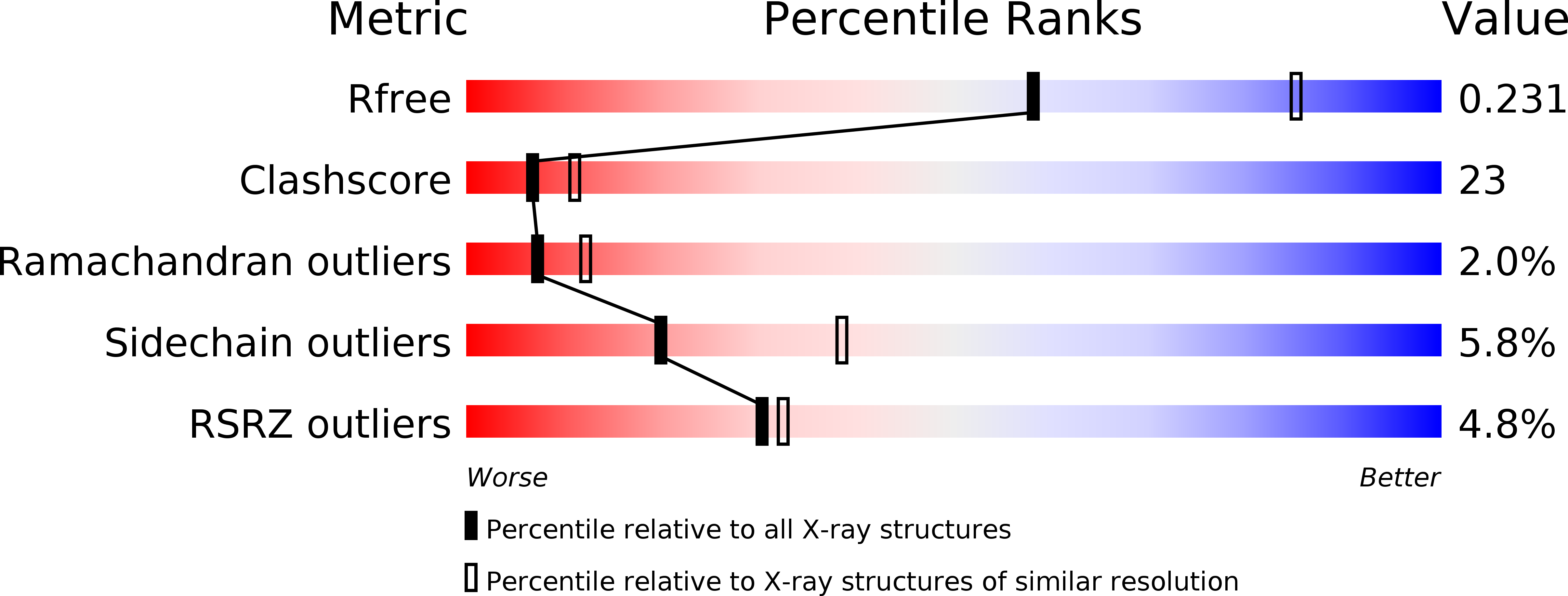
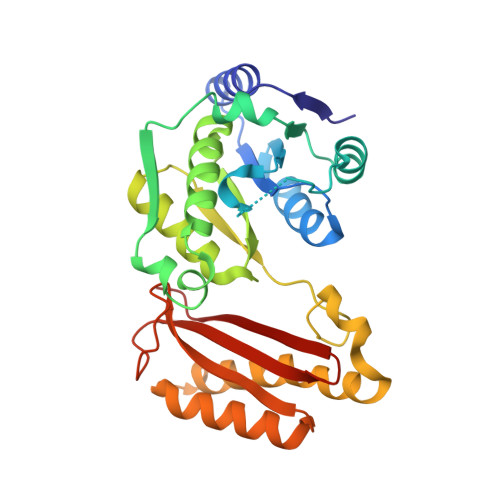
Substrate-induced closing of the active site revealed by the crystal structure of pantothenate synthetase from Staphylococcus aureus.
Satoh, A., Konishi, S., Tamura, H., Stickland, H.G., Whitney, H.M., Smith, A.G., Matsumura, H., Inoue, T.(2010) Biochemistry 49: 6400-6410
- PubMed: 20568730
- DOI: https://doi.org/10.1021/bi1004206
- Primary Citation of Related Structures:
3AG5, 3AG6 - PubMed Abstract:
Pantothenate synthetase (PS, EC 6.3.2.1) is the last enzyme in the pantothenate biosynthesis pathway, a metabolic pathway identified as a potential target for new antimicrobials. PS catalyzes the ATP-dependent condensation of pantoate and beta-alanine to form pantothenate. Here we report the overexpression, purification, enzyme assay, and tertiary structure of PS from Staphylococcus aureus. PS activity was experimentally confirmed, indicating a k(cat) value comparable to those of enzymes from other organisms. The structures of the apoenzyme and the reaction intermediate (pantoyl adenylate; PA) complex were determined by X-ray crystallography to resolutions of 2.5 and 1.85 A, respectively. Structural analysis indicated that the apoenzyme adopts an open and relatively mobile structure, while the complex structure is closed and entirely rigid. Structural comparison of the apoenzyme and the complex revealed how S. aureus PS undergoes open/close conformational change, and also determined the key interactions with the adenine ring of PA for a hinge bending domain closure. In the complex structure, PA and acetate are bound in the active site. We suggest that the acetate mimics the substrate beta-alanine. Therefore, the complex structure seems to represent a catalytic state poised for in-line nucleophilic attack on PA. These data also offer an alternative strategy for designing novel compounds that selectively inhibit PS activity.
Organizational Affiliation:
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, Japan.