An additional C-terminal loop in endonuclease IV, an apurinic/apyrimidinic endonuclease, controls binding affinity to DNA
Asano, R., Ishikawa, H., Nakane, S., Nakagawa, N., Kuramitsu, S., Masui, R.(2011) Acta Crystallogr D Biol Crystallogr 67: 149-155
- PubMed: 21358045
- DOI: https://doi.org/10.1107/S0907444910052479
- Primary Citation of Related Structures:
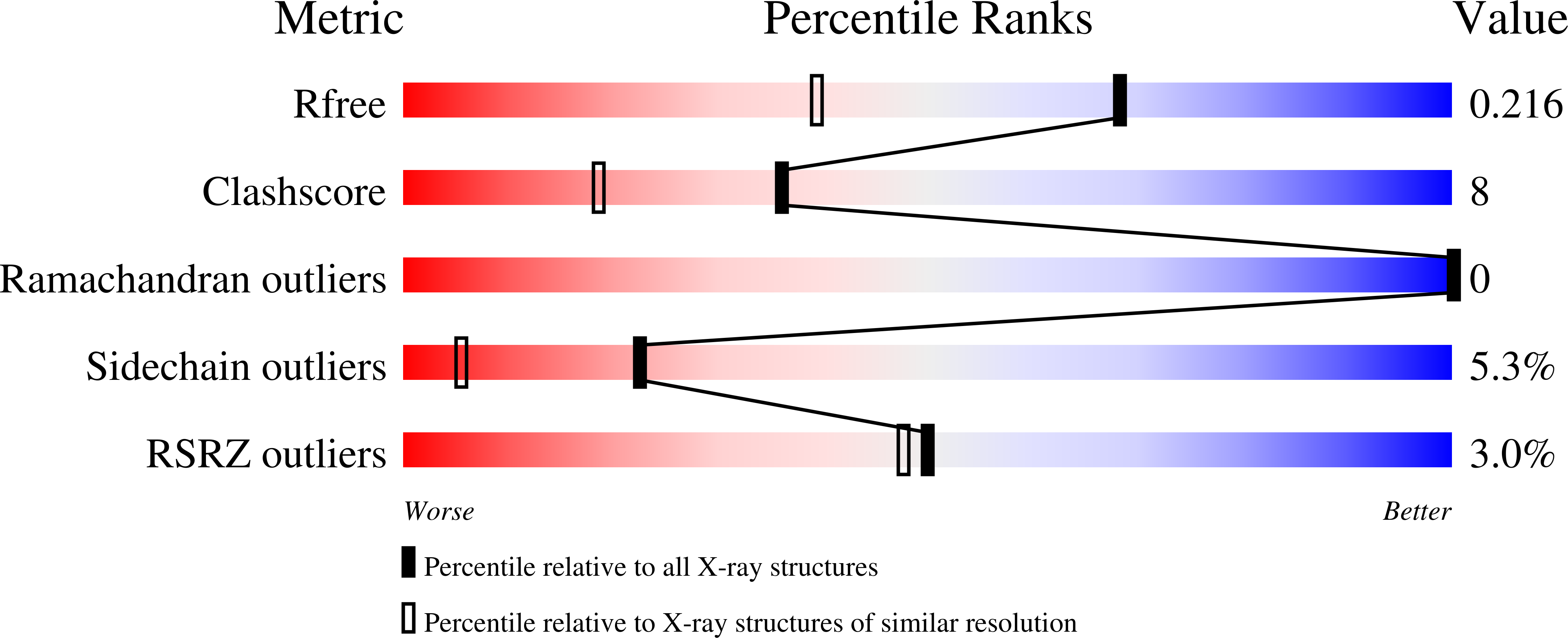
3AAL, 3AAM - PubMed Abstract:
Endonuclease IV (EndoIV) is an endonuclease that acts at apurinic/apyrimidinic (AP) sites and is classified as either long-type or short-type. The crystal structures of representative types of EndoIV from Geobacillus kaustophilus and Thermus thermophilus HB8 were determined using X-ray crystallography. G. kaustophilus EndoIV (the long type) had a higher affinity for double-stranded DNA containing an AP-site analogue than T. thermophilus EndoIV (the short type). Structural analysis of the two different EndoIVs suggested that a C-terminal DNA-recognition loop that is only present in the long type contributes to its high affinity for AP sites. A mutation analysis showed that Lys267 in the C-terminal DNA-recognition loop plays an important role in DNA binding.
Organizational Affiliation:
Department of Biological Sciences, Graduate School of Science, Osaka University, Toyonaka, Osaka, Japan.