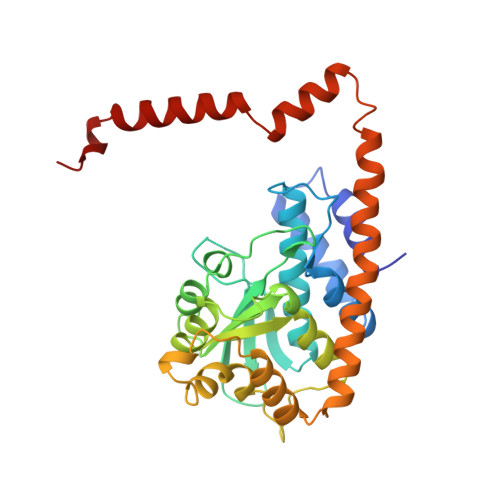
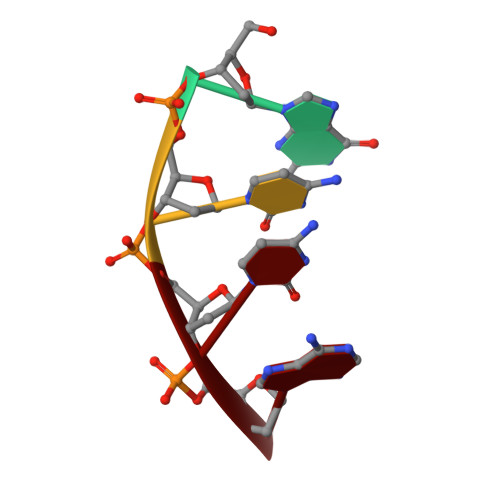
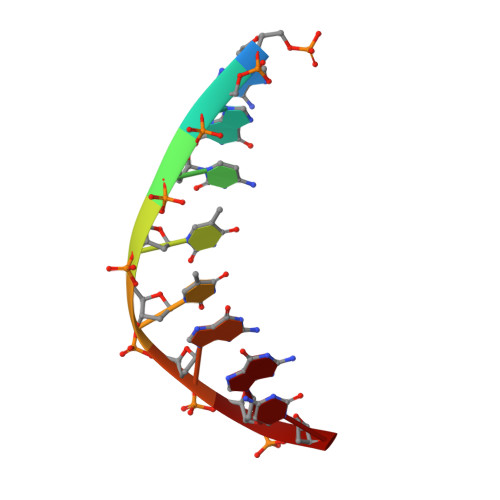
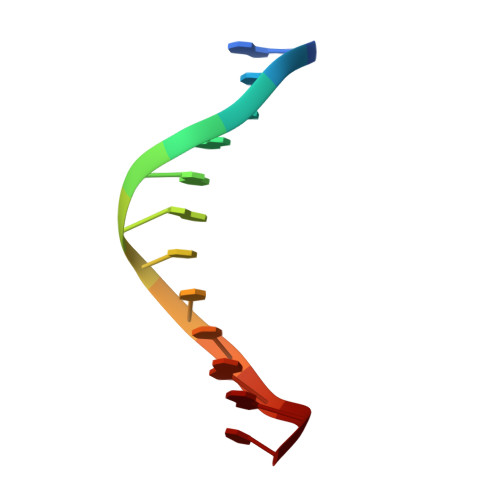
Structures of restriction endonuclease HindIII in complex with its cognate DNA and divalent cations
Watanabe, N., Takasaki, Y., Sato, C., Ando, S., Tanaka, I.(2009) Acta Crystallogr D Biol Crystallogr 65: 1326-1333
- PubMed: 19966419
- DOI: https://doi.org/10.1107/S0907444909041134
- Primary Citation of Related Structures:
2E52, 3A4K - PubMed Abstract:
The three-dimensional crystal structures of HindIII bound to its cognate DNA with and without divalent cations were solved at 2.17 and 2.00 A resolution, respectively. HindIII forms a dimer. The structures showed that HindIII belongs to the EcoRI-like (alpha-class) subfamily of type II restriction endonucleases. The cognate DNA-complex structures revealed the specific DNA-recognition mechanism of HindIII by which it recognizes the palindromic sequence A/AGCTT. In the Mg(2+) ion-soaked structure the DNA was cleaved and two ions were bound at each active site, corresponding to the two-metal-ion mechanism.
Organizational Affiliation:
Department of Biotechnology and Biomaterial Chemistry, Graduate School of Engineering, Nagoya University, Japan. nobuhisa@nagoya-u.jp