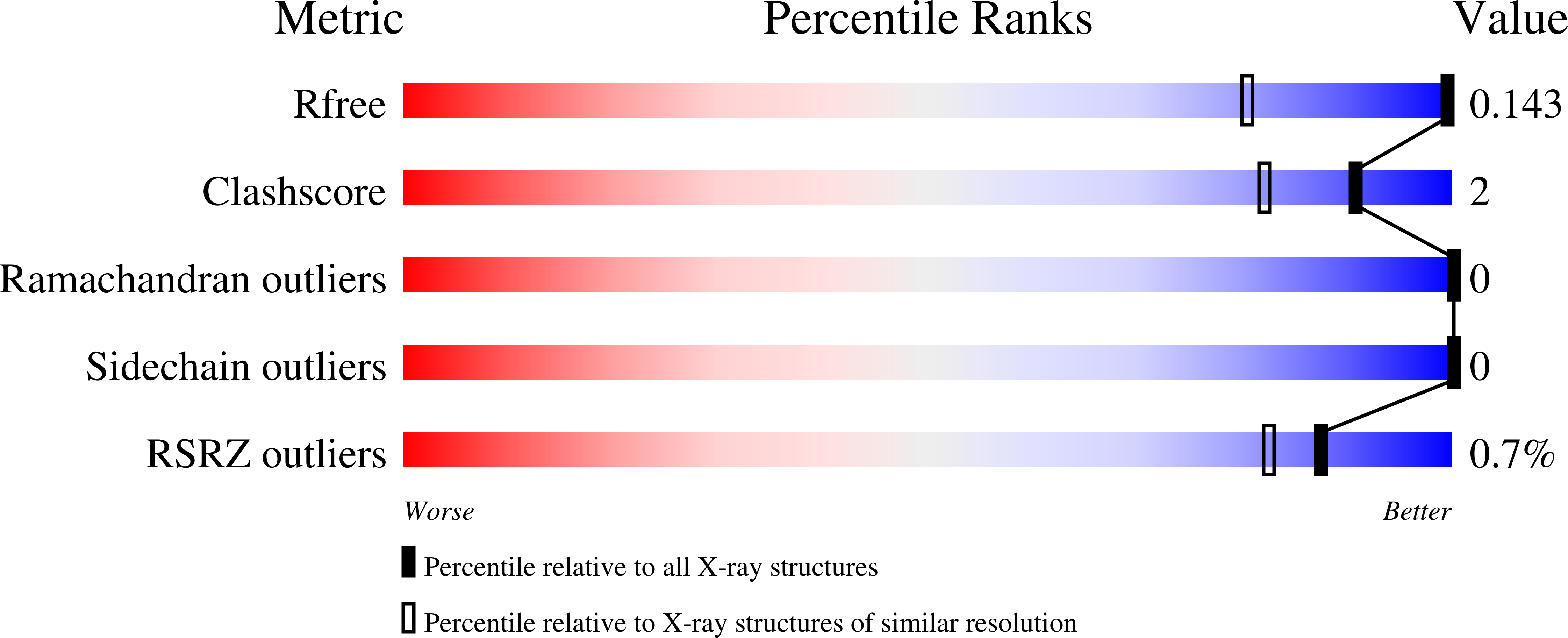
Stabilization of triple-helical structures of collagen peptides containing a Hyp-Thr-Gly, Hyp-Val-Gly, or Hyp-Ser-Gly sequence.
Okuyama, K., Miyama, K., Morimoto, T., Masakiyo, K., Mizuno, K., Bachinger, H.P.(2011) Biopolymers 95: 628-640
- PubMed: 21442606
- DOI: https://doi.org/10.1002/bip.21625
- Primary Citation of Related Structures:
3A0M, 3A1H, 3ADM - PubMed Abstract:
The single-crystal structures of three collagen-like host-guest peptides, (Pro-Pro-Gly)(4) -Hyp-Yaa-Gly-(Pro-Pro-Gly)(4) [Yaa = Thr, Val, Ser; Hyp = (4R)-4-hydroxyproline] were analyzed at atomic resolution. These peptides adopted a 7/2-helical structure similar to that of the (Pro-Pro-Gly)(9) peptide. The stability of these triple helices showed a similar tendency to that observed in Ac-(Gly-Hyp-Yaa)(10) -NH(2) (Yaa = Thr, Val, Ser) peptides. On the basis of their detailed structures, the differences in the triple-helical stabilities of the peptides containing a Hyp-Thr-Gly, Hyp-Val-Gly, or Hyp-Ser-Gly sequence were explained in terms of van der Waals interactions and dipole-dipole interaction between the Hyp residue in the X position and the Yaa residue in the Y position involved in the Hyp(X):Yaa(Y) stacking pair. This idea also explains the inability of Ac-(Gly-Hyp-alloThr)(10) -NH(2) and Ac-(Gly-Hyp-Ala)(10) -NH(2) peptides to form triple helices. In the Hyp(X):Thr(Y), Hyp(X):Val(Y), and Hyp(X):Ser(Y) stacking pairs, the proline ring of the Hyp residues adopts an up-puckering conformation, in agreement with the residual preference of Hyp, but in disagreement with the positional preference of X in the Gly-Xaa-Yaa sequence.
Organizational Affiliation:
Department of Macromolecular Science, Graduate School of Science, Osaka University, Osaka 560-0043, Japan. okuyamak@chem.sci.osaka-u.ac.jp