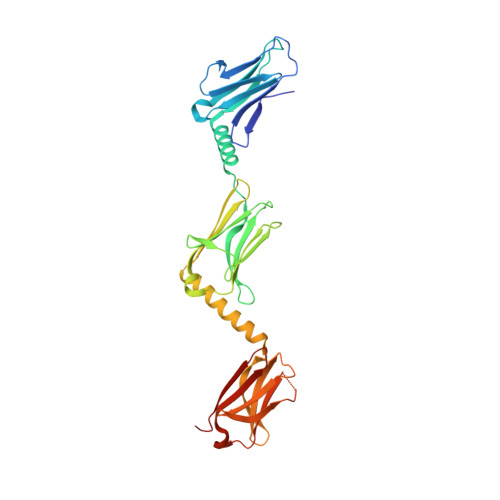
Superhelical Architecture of the Myosin Filament-Linking Protein Myomesin with Unusual Elastic Properties.
Pinotsis, N., Chatziefthimiou, S.D., Berkemeier, F., Beuron, F., Mavridis, I.M., Konarev, P.V., Svergun, D.I., Morris, E., Rief, M., Wilmanns, M.(2012) PLoS Biol 10: 1261
- PubMed: 22347812
- DOI: https://doi.org/10.1371/journal.pbio.1001261
- Primary Citation of Related Structures:
2Y23, 2Y25, 3RBS - PubMed Abstract:
Active muscles generate substantial mechanical forces by the contraction/relaxation cycle, and, to maintain an ordered state, they require molecular structures of extraordinary stability. These forces are sensed and buffered by unusually long and elastic filament proteins with highly repetitive domain arrays. Members of the myomesin protein family function as molecular bridges that connect major filament systems in the central M-band of muscle sarcomeres, which is a central locus of passive stress sensing. To unravel the mechanism of molecular elasticity in such filament-connecting proteins, we have determined the overall architecture of the complete C-terminal immunoglobulin domain array of myomesin by X-ray crystallography, electron microscopy, solution X-ray scattering, and atomic force microscopy. Our data reveal a dimeric tail-to-tail filament structure of about 360 Å in length, which is folded into an irregular superhelical coil arrangement of almost identical α-helix/domain modules. The myomesin filament can be stretched to about 2.5-fold its original length by reversible unfolding of these linkers, a mechanism that to our knowledge has not been observed previously. Our data explain how myomesin could act as a highly elastic ribbon to maintain the overall structural organization of the sarcomeric M-band. In general terms, our data demonstrate how repetitive domain modules such as those found in myomesin could generate highly elastic protein structures in highly organized cell systems such as muscle sarcomeres.
- European Molecular Biology Laboratory Hamburg, Hamburg, Germany.
Organizational Affiliation: