Solution structure of NapD, a private chaperone of periplasmic nitrate reductase NapA/B, in complex with NapA1-35 signal peptide.
Minailiuc, O.M., Ekiel, I., Cheng, J., Milad, M., Gandhi, S., Larocque, R., Cygler, M., Matte, A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein napD | 90 | Escherichia coli | Mutation(s): 0 Gene Names: napD, b2207, JW2195 | 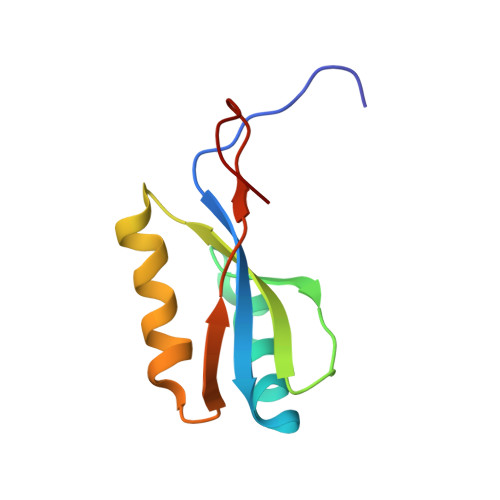 | |
UniProt | |||||
Find proteins for P0A9I5 (Escherichia coli (strain K12)) Explore P0A9I5 Go to UniProtKB: P0A9I5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0A9I5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Periplasmic nitrate reductase precursor | 35 | Escherichia coli | Mutation(s): 0 Gene Names: napA, b2206, JW2194 EC: 1.7.99.4 (PDB Primary Data), 1.9.6.1 (UniProt) | 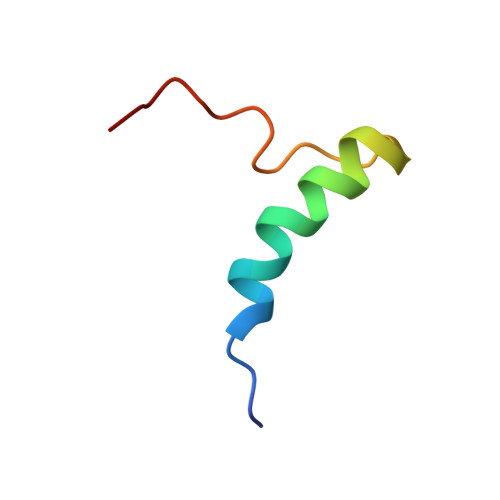 | |
UniProt | |||||
Find proteins for P33937 (Escherichia coli (strain K12)) Explore P33937 Go to UniProtKB: P33937 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P33937 | ||||
Sequence AnnotationsExpand | |||||
| |||||